Copy link
Whole Blood Transfusions
Last updated: 02/21/2023
Key Points
- The Association for Advancement of Blood and Biotherapies (AABB) recommends using low-titer group O whole blood (LTOWB) as a part of the prehospital and hospital care for acute hemorrhage.1
- Compared to component therapy (CT), whole blood has several advantages including a better hemostatic profile, less volume, less additives, and decreased exposure to donors.
- LTOWB can be safely used in women of childbearing age group.
Introduction
- Group O whole blood has been safely used for hemorrhage since the 1800s. The United States (US) Army used whole blood in both the world wars, the Korean war, and the Vietnam war.2,3
- However, blood banks in the US transitioned to CT in the 1970s and 80s to conserve resources and provide specific components for isolated deficits in different patient populations.2 CT involves dividing whole blood into packed red blood cells (RBC), fresh frozen plasma (FFP), platelets, and cryoprecipitate.
- More recently, balanced resuscitation for acute hemorrhage with varying ratios of RBCs, FFP, and platelets (1:1:1 or 2:1:1) have evolved to mimic whole blood.
- The use of whole blood in the recent military operations in Iraq and Afghanistan have also renewed the interest in its use by the civilian trauma community.
- The AABB now recommends using LTOWB as a part of the prehospital and hospital care for acute hemorrhage, even in pregnant patients.1
Description of Whole Blood
- Whole blood is collected in citrate-phosphate dextrose (CPD) storage solution and stored between 1°C and 6°C. Whole blood can last up to 21 days in CPD and up to 35 days in citrate-phosphate dextrose-adenine (CPDA) solution.2,3
- Most US centers limit the use of whole blood to 14-21 days, and it doesn’t need to be agitated or frozen.3
- Current whole blood donors are male to reduce the risk of transfusion-related acute lung injury. Most centers use group O positive male donors for all males and females over the age of 50 years.
- Group O negative whole blood, if available, is usually reserved for females of child-bearing age.
- LTOWB is group O whole blood with low levels of anti-A and anti-B immunoglobulins M. There is no current standard definition for low titer. Low titers are defined as less than 1:256. Some centers use a higher threshold for titers (1:50).3
- Table 1 lists the composition of whole blood compared to CT.
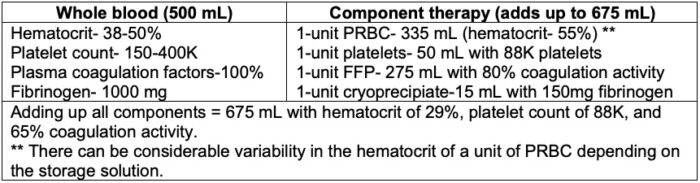
Table 1. Composition of whole blood compared to CT. Adapted from Hanna M, et al. The use of whole blood transfusion in trauma. Curr Anesthesiol Rep. 2022; 12(2):234-9.3
Advantages of Whole Blood versus Components1-3
- Simplifies the logistics of transfusing a bag of whole blood vs. 2-3 separate components.
- Whole blood is more concentrated and has less total volume (see Table 1).
- Whole blood contains fewer additives compared to CT.
- Whole blood has a better hemostatic profile compared to CT (see Table 1).
- Cold stored platelets in whole blood have better hemostatic function.
- Cold-stored platelets in whole blood have a longer shelf-life vs. room-temperature platelets.
- Since whole blood is refrigerated, transfusing whole blood reduces bacterial contamination of platelet-containing products.
- Transfusing whole blood reduces the incidence of ABO-incompatibility and clerical errors.
- During a massive transfusion, whole blood can be given via a warmed rapid infuser, whereas platelets must be given at room temperature, which requires a separate administration.
- Transfusing whole blood reduces the patient’s exposure to donors.
Limitations/Special Considerations
Costs
- Initiating a whole blood program in addition to the already established CT program in blood banks can be very expensive.3
Potential for Wastage
- Most US centers limit LTOWB storage only for 14-21 days, which can result in significant wastage if the supply and demand are not matched.3
Limited Donor Pool
- Currently, group O positive males are used as donors, which reduces the donor pool.3 Finding an adequate number of O negative donors is even more challenging. Only about 3% of donors are eligible to donate Rh-negative LTOWB.
Risk of Rh-alloimmunization
- The administration of Rh-positive LTOWB to a Rh-negative woman in the childbearing age group may induce alloimmunization that may cause hemolytic reactions to subsequent transfusions or induce hemolytic disease of the fetus and newborn (HDFN) in subsequent pregnancies. The risk of HDFN ranges from 0.3-6.5%, where the anti-D antibodies can cross the placenta and induce hemolysis of fetal red cells. This can be further minimized by administering Rh-Immunoglobulin (Rh-Ig) to the mother.3
- Some researchers believe that the benefits of administering LTOWB to a female trauma patient outweigh the risks of her developing alloimmunization, surviving the massive transfusion, producing anti-Rh antibodies, and subsequently getting pregnant with a Rh-positive fetus.
- In a mathematical model based on one Rh negative woman receiving a LTOWB in 30 months, it was estimated that it would take 3000 months (or 250 years) for 100 Rh negative women to receive LTOWB, and between 3 and 30 of them would develop alloimmunization without the administration of Rh-Ig.4 Rh-Ig administration would further decrease the risk to 1%. During the same time, it was estimated that 500 women would die from hemorrhage without a LTOWB transfusion.
Hemolytic Transfusion Reactions
- Administering whole blood could potentially lead to anti-A and anti-B antibodies causing hemolytic transfusion reactions. This risk is reduced by using LTOWB. Most institutions also limit the total volume of whole blood that a patient can receive at a time.
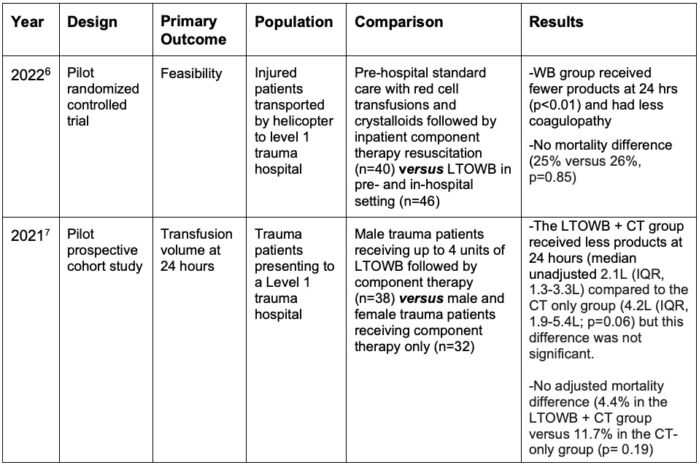
Table 2. Randomized Trial Results
Perioperative Use of Whole Blood
- While whole blood is used primarily in the military setting and in trauma patients, its use is expanding to other patient populations.8
- Prehospital setting: The use of LTOWB in ambulances and helicopters by paramedics for massively bleeding patients is gaining popularity.4,6
- Obstetrics: The early experience of using LTOWB for postpartum hemorrhage in 7 patients has been reported from two US institutions. Intermountain Medical Center in Utah used emergency release, uncrossmatched LTOWB as a part of a massive transfusion protocol and the University of Texas San Antonio group used crossmatched LTOWB for high-risk obstetric patients with known placental abnormalities.9
- Transplant and cardiac surgery: The successful use of whole blood has also been reported for liver transplantation and cardiac surgery.8
References
- Yazer MH, Spinella PC, Bank EA, et al. THOR-AABB Working Party recommendations for a pre-hospital blood product transfusion program. Prehosp Emerg Care. 2022;26(6):863-75. PubMed
- Spinella PC, Cap AP. Whole blood: back to the future. Curr Opin Hematol. 2016;23(6):536-42. PubMed
- Hanna M, Knittel J, Gillihan J. The use of whole blood transfusion in trauma. Curr Anesthesiol Rep. 2022; 12(2):234-9. PubMed
- McGinity AC, Zhu CS, Greebon L, et al. Prehospital low-titer cold-stored whole blood: Philosophy for ubiquitous utilization of O-positive product for emergency use in hemorrhage due to injury. J Trauma Acute Care Surg. 2018;84(6S Suppl 1): S115-9. PubMed
- Yazer M, Triulzi D, Sperry J, et al. Rate of RhD-alloimmunization after the transfusion of RhD-positive red blood cell containing products among injured patients of childbearing age: single center experience and narrative literature review. Hematology. 2021;26(1):321-7. PubMed
- Guyette FX, Zenati M, Triulzi DJ, et al. Prehospital low titer group O whole blood is feasible and safe: Results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2022;92(5):839-47. PubMed
- Siletz AE, Blair KJ, Cooper RJ. A pilot study of stored low titer group O whole blood + component therapy versus component therapy only for civilian trauma patients” J Trauma Acute Care Surg. 2021;91(4):655-62. PubMed
- Stephens CT, de Haan JB, Jonna S, et al. Whole blood in modern anesthesia practice. Adv Anesth. 2020;38: 115-29. PubMed
- Morris DS, Braverman MA, Correan J, et al. Whole blood for postpartum hemorrhage: early experience at two institutions. Transfusion. 2020;60: S31-35. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.