Copy link
Understanding Bias in Research
Last updated: 01/06/2025
Key Points
- Bias can underestimate, overestimate, or completely reverse the true association between exposure and outcome, which impacts the reliability of study findings.
- Bias can occur at any stage of research (i.e., design, data collection, analysis, or reporting).
- Addressing biases through rigorous design, data collection, and advanced statistical techniques helps improve the accuracy and applicability of research.
Conceptual Framework of Bias in Statistics
Definition of Bias
- Bias is an error that causes results to differ from the true value.1,2
- This ultimately leads to incorrect conclusions being made regarding the relationship between exposure and effect.2
- It also weakens the accuracy of a study’s results, which makes it harder to extrapolate and apply these findings to other populations.2
- Bias can influence the accuracy of study results in different ways, depending on the direction in which it skews findings1 (Table 1).
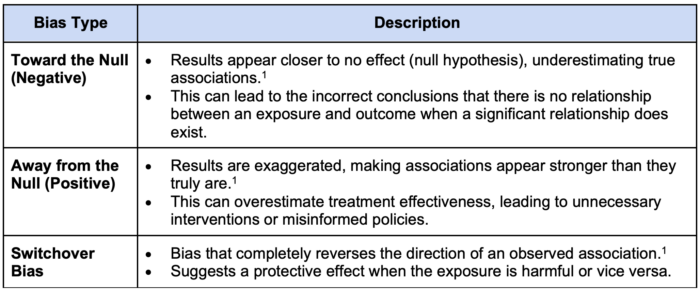
Table 1. Types of bias by direction
Importance of Recognizing Bias
- Failure to address bias can result in flawed policy and compromised patient outcomes.3
- Anesthesiology research can yield more accurate and clinically relevant findings by addressing bias through comprehensive design, data collection, and advanced statistical methods.
Classification of Bias
- Bias can occur at various stages of research, from study design and participant selection to data collection, analysis, and reporting.1,2
- Classifying bias helps identify the source of error and allows researchers to create strategies for minimizing or eliminating its impact.
- The primary types of bias include selection bias, information bias, performance bias, attrition bias, confounding, and publication bias.1
Primary Types of Biases (Table 2)
1. Selection Bias: Occurs when participants are not representative of the target population.1,2
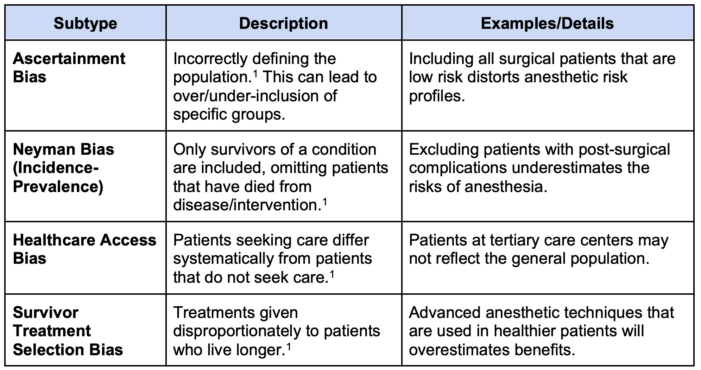
Table 2. Forms of selection bias
2. Information Bias: Results from systematic errors in data collection or measurement1,2,4 (Table 3).
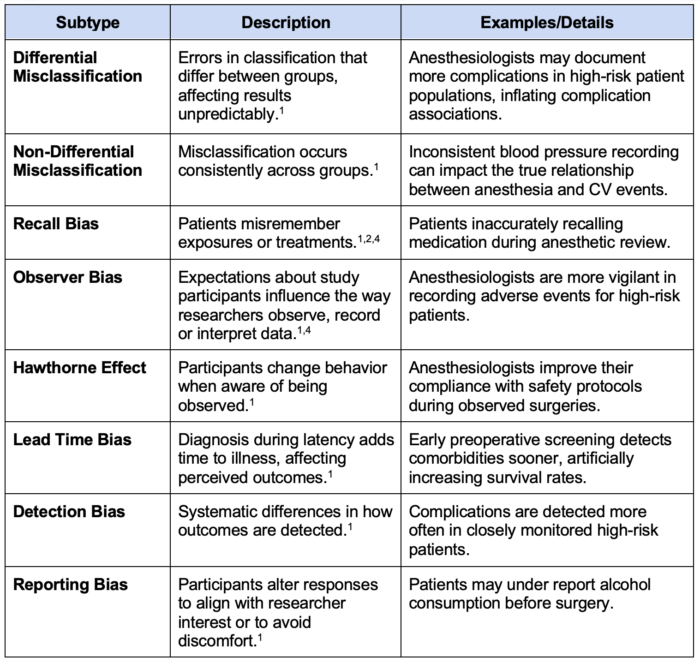
Table 3. Types of information bias
3. Performance Bias: Occurs when there are differences in care between groups (i.e., other than the intervention being studied)2
- To minimize performance bias, investigators can group patients by an anesthesiologist, assigning all patients treated by the same provider to one study group.2
4. Attrition Bias: Results from unequal loss to follow-up between study groups4,5
- In anesthesiology trials, patients lost to follow-up due to complications may skew results.
- Sensitivity analyses can help address this bias by testing the impact of missing data.5
5. Confounding: Occurs when an external variable influences both the exposure and the outcome, creating a false association1,2,4 (Table 4).
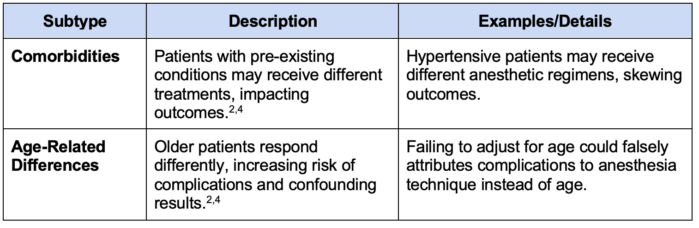
Table 4. Types of confounding bias
6. Publication Bias: Occurs when studies with positive or statistically significant results are more likely to be published than those with negative or null results1,2
a. This leads to an overestimation of treatment effects.
Addressing Bias in Anesthesiology Research
- In anesthesiology research, minimizing bias is essential to produce accurate, reliable, and generalizable results.
- The strategies highlighted below (Figure 1) are essential for reducing different types of bias and enhancing the validity of research findings.

Figure 1. Strategies to enhance research quality
References
- Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635-41. PubMed
- Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126(2):619-25. PubMed
- Gopal DP, Chetty U, O'Donnell P, et al. Implicit bias in healthcare: clinical practice, research and decision making. Future Healthc J. 2021;8(1):40-8. PubMed
- Vetter TR, Mascha EJ. Bias, Confounding, and Interaction: Lions, Tigers, and Bears, Oh My! Anesth Analg. 2017;125(3):1042-48. PubMed
- Nunan D, Aronson J, Bankhead C. Catalogue of bias: attrition bias. BMJ Evid Based Med. 2018;23(1):21-2. PubMed
- Mozetic V, Leonel L, Leite Pacheco R, et al. Reporting quality and adherence of randomized controlled trials about statins and/or fibrates for diabetic retinopathy to the CONSORT checklist. Trials. 2019;20(1):729. PubMed
- Depaoli S, Winter SD, Visser M. The importance of prior sensitivity analysis in Bayesian statistics: Demonstrations using an interactive shiny app. Front Psychol. 2020; 11:608045. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.