Copy link
Trauma-Induced Coagulopathy
Last updated: 02/16/2023
Key Points
- Coagulation abnormalities are common following major trauma, and trauma-induced coagulopathy (TIC) is an independent risk factor for death.1
- TIC can manifest as a spectrum of phenotypes. Early TIC is dominated by acute blood loss with associated shock, impaired clot formation, and fibrinolysis; late systemic TIC phenotype presents with thrombotic-antifibrinolytic, mirroring DIC phenotypes.2
- Several pathophysiologic mechanisms underlie TIC, including hemorrhagic shock, tissue injury, endothelial dysfunction, platelet dysfunction, inappropriate thrombin generation, fibrinogen depletion, and dysregulated fibrinolysis.2,3
Introduction
- TIC describes abnormal coagulation processes that are attributable to trauma.1,2
- Response to trauma is a complex, dynamic process in which risk can shift from bleeding to thrombosis depending on the injury pattern, treatment administered, individual responses, and comorbidities.
- TIC is present shortly after injury and before any resuscitative efforts have been initiated, meaning the coagulopathy cannot be attributed to the dilutional effects of resuscitative fluids alone.1
- TIC can manifest as a spectrum of phenotypes. Early TIC (generally within 6h of injury) is characterized by a hypocoagulable state, resulting in bleeding and shock. Late TIC (usually more than 24 hours after injury) is characterized by a hypercoagulable state associated with venous thromboembolism and multiple organ failure.2 Transition between the two states can occur within minutes or hours or be delayed for days.2
- The incidence of TIC is around 25% of severely injured patients, with an associated mortality of 35-50%.1,2 Trauma patients with TIC have nearly a 4-fold increased mortality than those without TIC.3 Children generally develop TIC less frequently and later than adults. Older adults are more vulnerable to TIC than younger adults.2
- Risk factors for TIC include2
- Severe tissue injury
- Shock
- Penetrating injury
- Metabolic acidosis
- Long pre-hospital times
- Large volume of crystalloid
- Laboratory diagnosis of TIC is based on coagulation abnormalities detected by conventional tests (platelet count, fibrinogen level, PT, and aPTT) or viscoelastic hemostatic assays (VHA), which include thromboelastography (TEG) and rotational thromboelastometry (ROTEM).2
Pathophysiology
- Physiological response to minor trauma based on endothelial activation becomes a pathological response in massive trauma that leads to endothelial damage (Figure 1).3
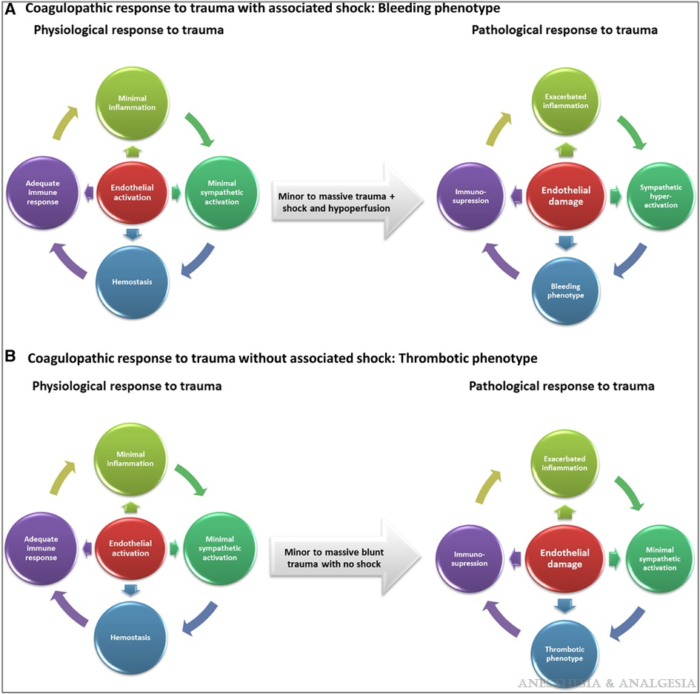
Figure 1. Coagulative response to trauma. Reproduced with permission from Duque P et al. Anesth Analg. 2020.3
- Several pathophysiologic mechanisms underlie TIC, including hemorrhagic shock, tissue injury, endothelial dysfunction, platelet dysfunction, inappropriate thrombin generation, fibrinogen depletion, and dysregulated fibrinolysis.2,3
- Tissue injury and hemorrhagic shock synergistically activate the endothelium, platelets, and the immune system.
- This generates an array of mediators (damage-associated molecular patterns, tumor necrosis factor-alpha, tissue plasminogen activator, activated protein C, plasminogen activator inhibitor-1) that reduce fibrinogen, impair platelet function, and compromise thrombin generation.
- Insufficient thrombin concentration results in clots with diminished stability and prone to fibrinolysis, which ultimately results in inadequate clot formation for hemostasis.
- Increased fibrinolysis via increased plasmin generation further compromises hemostatic capacity by shifting the balance of the fibrinolytic system, promoting the premature breakdown of fibrin clots as well as fibrinogen degradation.
- The mechanisms of TIC are accentuated by the “Lethal Triad” of coagulopathy, metabolic acidosis, and hypothermia.2 Some authors have suggested including hypocalcemia and renaming it the lethal diamond.2
- Hypothermia (temperature <36°C) worsens coagulopathy by reducing the production of thrombin, reducing the activity of circulating clotting factors, and decreased platelet function.
- Acidosis worsens coagulopathy by reducing clotting factor activity in both intrinsic and extrinsic pathways, as seen by elevated aPTT and PT values at lower pH.
Targeted Therapy
- Patients with severe tissue injuries presenting without shock are hypercoagulable and at high risk for thrombosis. Thromboprophylaxis should be initiated to prevent thrombosis. On the other hand, patients with severe tissue injuries presenting in shock develop hypocoagulability and hyperfibrinolysis, which should be treated with early hemostatic goal-directed resuscitation (Figure 2).3
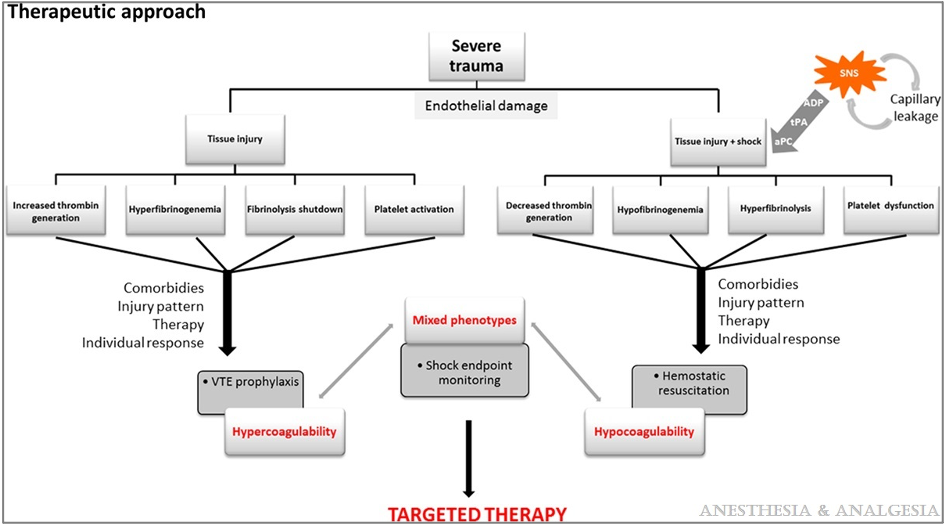
Figure 2. Therapeutic approach to severe tissue injury. Reproduced with permission from Duque P et al. Anesth Analg. 2020.3
- Management priorities:
- Mechanically stopping the bleeding, using tourniquets and direct pressure in the field and rapid access to a capable trauma surgeon.
- Reversing hypovolemic shock by restoring circulating blood volume in a balanced 1:1:1 ratio.
- Restoring clotting hemostasis by administering the right blood products to the right patient at the right time.2
- Fluids administered in the prehospital setting can help but also potentially harm the patient, avoiding resuscitation using large volume crystalloids.
- Plasma resuscitation en route reduces mortality in patients with blunt force trauma and transport times longer than 20 minutes.2
- Early administration of tranexamic acid safely reduces the risk of death in bleeding trauma patients, although treatment beyond 3 hours of injury is unlikely to be effective.4
- Loading dose of 1 g over 10 min, followed by an infusion of 1 g over 8 hr.
- Calcium is an essential part of the coagulation cascade and must be monitored and corrected promptly to achieve hemostasis.1
- Avoid giving bicarbonate unless severe acidosis is present.2
- Decreased fibrinogen levels due to hyperfibrinolysis should be restored with cryoprecipitate.
- Ongoing coagulation monitoring using VHA-guided goal-directed resuscitation reduced mortality by almost 50%.2
- Thromboprophylaxis should be administered as soon as possible after the bleeding risk has subsided due to patient immobility and hypercoagulable state.2
References
- McGlinch, B. Anesthesia for trauma & emergency surgery. Butterworth, J, Mackey, D. Morgan & Mikhail’s Clinical Anesthesiology. 6th edition. Lange; 2018: 824-27.
- Moore EE, Moore HB, Kornblith LZ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers 2021;7: 30. PubMed
- Duque P, Mora L; Levy JH. et al. Pathophysiological response to trauma-induced coagulopathy: A comprehensive review. Anesth Analg. 2020;130(3): 654-664. PubMed
- Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1-79. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.