Copy link
Transdermal Fentanyl
Last updated: 09/14/2023
Key Points
- Transdermal fentanyl is primarily indicated for cancer-related pain as an alternative to oral morphine.
- Transdermal therapeutic system (TTS) delivery of fentanyl can also be utilized when a patient is unable to swallow oral morphine or other long-acting opioids or when concerns exist about renal function and the ability to excrete morphine metabolites.
- Transdermal fentanyl takes approximately 20 hours to reach its peak analgesic effect.
- Patients should have a constant awareness of body temperature and the effects of external heat sources that can lead to an increased rate of dermal absorption.
Introduction
- Fentanyl is a synthetic opioid that is 100 times more potent than morphine. It has a highly lipophilic structure, which allows it to rapidly cross the blood-brain barrier. It can be administered via intravenous, intranasal, sublingual, buccal mucosal patch, transdermal, and oral lozenge routes.1
- Fentanyl’s low molecular weight, high potency, and lipid solubility make it ideal for transdermal use.
Mechanism of Action
- After absorption from the transdermal route into the systemic circulation, fentanyl exerts its analgesic effects by acting as a mu-opioid receptor agonist in the brain and spinal cord. To a lesser extent, it also works on the delta and kappa receptors.
Pharmacokinetics
- Fentanyl is released at a nearly constant rate from the matrix system embedded into the patch. Given its high-lipid solubility, it enters the epidermal lipids and forms a depot at the dermal-epidermal junction. This slowly dissolves in the hydrophilic dermis and enters the cutaneous circulation. There is a gradual increase in serum concentrations over the next 24 hours before it reaches a steady state.2
- Although fentanyl can be detected in the serum about 1-2 hours after initial administration, therapeutic serum fentanyl concentrations are not achieved until 12-16 hours after application.2 Peak analgesic effect requires an average of 20 hours; however, up to 72 hours may be needed in certain patient populations.3
- Transdermal delivery of fentanyl bypasses first-pass drug metabolism by the liver. There is wide variability in the rate of absorption of transdermal fentanyl, which can be affected by the thickness of the skin and subcutaneous fat layer, and subcutaneous perfusion.3,4
- Elevated body temperatures can accelerate the release of fentanyl from the patch itself or its distribution from subcutaneous fat deposits.3,4 Therefore, patients should be instructed to avoid sunbathing, using hot tubs, saunas, heating blankets, heating lamps, etc.4
- Duration of action: If the patch is removed and not replaced, some effects may last between 72 and 96 hours due to an extended half-life. Fentanyl concentrations decrease by approximately 50% in the first 20 to 27 hours following removal.3
Indications
- Transdermal fentanyl is primarily indicated for cancer-related pain as an alternative to oral morphine.5 It is used mostly when patients are unable to swallow oral morphine or other long-acting opioids or when concerns exist about renal function and the ability to excrete morphine metabolites.2
- Current studies are still examining the utility of transdermal fentanyl in chronic nonmalignant pain.5
Dosing Recommendations
- Common dosages of TTS fentanyl include 12.5, 25, 37.5, 50, 75, and 100 µg/hour.3
- After calculating the patient’s 24-hour total opioid requirements, the morphine milliequivalents can be converted to fentanyl.3 (Table 1)
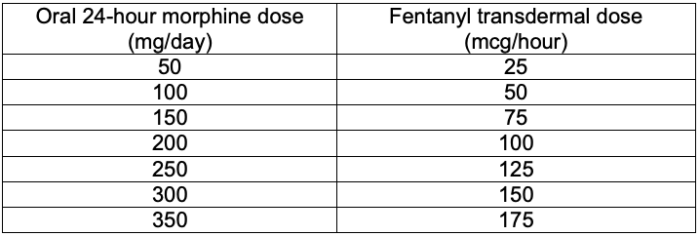
Table 1. Morphine to transdermal fentanyl dosage conversion chart. Adapted from McPherson ML. Demystifying conversion calculations: A guide for effective dosing. 2nd ed. Bethesda, MD: American Society of Health-System Pharmacists; 20166
- When converting from a continuous intravenous (IV) fentanyl infusion to a transdermal patch, a 1:1 (IV: transdermal) ratio has been utilized safely in cancer patients.7 The continuous IV infusion was decreased by 50% six hours after applying the patch and discontinued six hours later.
- Titrations of transdermal fentanyl dosages should be made every 72 hours when the patch is to be replaced. Ensuring immediate release medications for breakthrough pain are available is essential for quantifying the amount needed for future dosage increases.7,8
- Decreasing or discontinuing transdermal fentanyl is recommended to go slowly and titrate down by 25% every 2-4 weeks. Swift or abrupt discontinuation can lead to severe opioid withdrawals.3
Common Side Effects
- Due to the concern for postoperative respiratory depression, a transdermal fentanyl patch should be removed and or held in the perioperative period. Short-acting opioids can be utilized in the perioperative period with the option of restarting the transdermal fentanyl patch after 1-2 days postoperatively. There is no need to titrate down before removing the patch. Additionally, there is a relatively low risk of hypoventilation in cancer patients outside of the perioperative period.5
- Other notable adverse side effects include sedation, nausea, vomiting, and constipation. Transdermal fentanyl has been shown to have fewer gastrointestinal side effects than other opioids, including oral morphine.5
- Transdermal fentanyl with concomitant usage of cytochrome P450 3A4 inhibitors such as amiodarone, diltiazem, and fluconazole can potentially increase the serum levels of fentanyl.3
Safety Issues
- Due to the presence of the drug in an adhesive formulary, there is a risk of leaking and potential extrication for illicit drug use.3
- Patients are advised to not cut or alter the patch in any way.3
- Patients should have a constant awareness of body temperature and the effects of external heat sources that can lead to an increased rate of dermal absorption.3
- Proper disposal of patches is key to preventing potential abuse and unintended administration of the drug upon removal of the patch.3
- To properly dispose of the patch, patients are instructed to simply fold the two adhesive sides of the patch together and flush it down the toilet.3
References
- Taylor KP, Singh K, Goyal A. Fentanyl transdermal. In: StatPearls (Internet). Treasure Island, FL; StatPearls Publishing; 2022. Link
- Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5(4):230-41. PubMed
- Duragesic Data Sheet. Janssen Pharmaceuticals, Titusville, NJ. Accessed: September 14, 2023. Link
- Kuip EJ, Zandvliet ML, Koolen SL, et al. A review of factors explaining variability in fentanyl pharmacokinetics; focus on implications for cancer patients. Br J Clin Pharmacol. 2017;83(2):294-313. PubMed
- Schumacher M, Fukuda K. Chapter 24: Opioids. In: Miller RD, et al.(eds). Miller’s Anesthesia. 9th ed. Philadelphia, PA; Churchill Livingstone Elsevier; 2020:680-741.
- McPherson ML. Demystifying conversion calculations: A guide for effective dosing. 2nd ed. Bethesda, MD: American Society of Health-System Pharmacists; 2016.
- Kornick CA, Santiago-Palma J, Khojainova N, et al. A safe and effective method for converting cancer patients from intravenous to transdermal fentanyl. Cancer. 2001;92(12):3056-61. PubMed
- Kaye AD, Menard BL, Ehrhardt KP, et al. Consensus perioperative management best practices for patients on transdermal fentanyl patches undergoing surgery. Curr Pain Headache Rep. 2019;23(7):50. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.