Copy link
Transcatheter Aortic Valve Replacement
Last updated: 07/26/2024
Key Points
- Transcatheter aortic valve replacement/implantation (TAVR/TAVI) is a viable alternative to surgical aortic valve replacement (SAVR), particularly in patients with prohibitive surgical risk.
- There are several periprocedural and postoperative complications associated with TAVR, including aortic annular dissection/rupture, coronary obstruction, cardiac tamponade, device migration, high-grade atrioventricular block, and cerebrovascular injury.
Indications1
- Indications for aortic valve replacement (surgical or transcatheter) in the setting of aortic stenosis (AS) include:
- Symptomatic severe high-gradient AS
- Asymptomatic patients with severe AS and ejection fraction < 50%
- Severe or moderate AS when undergoing other cardiac surgery
- Asymptomatic severe AS and low surgical risk
- Symptomatic with low-flow/low-gradient severe AS
- TAVR is approved for the following conditions:
- Low to prohibitive surgical risk patients with severe AS
- Valve-in-valve procedures for failed bioprosthetic valves
- Recommendations for SAVR vs. TAVR are listed in Table 1.
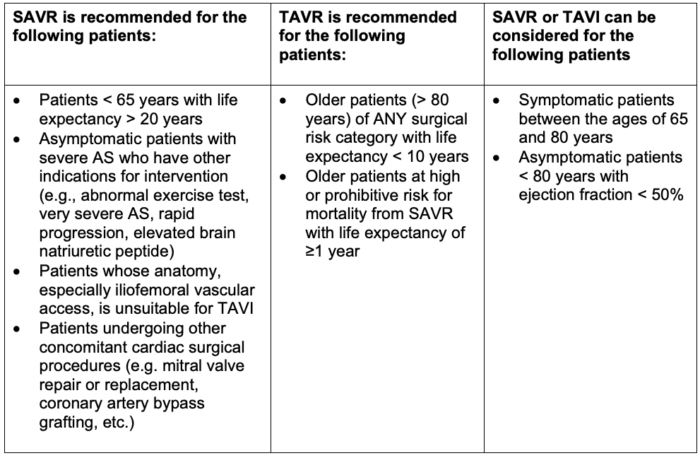
Table 1. Recommendations for SAVR vs TAVR
- Contraindications for TAVR/TAVI are listed in Table 2.
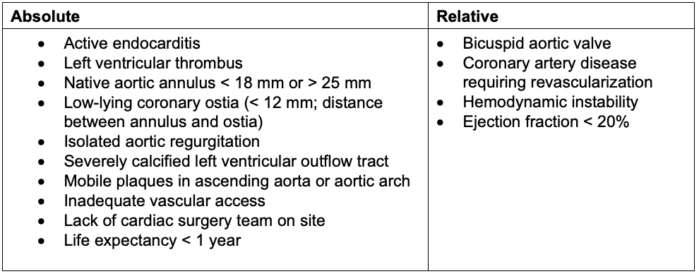
Table 2. Contraindications to TAVR/TAVI
Procedure1
- TAVR requires the use of fluoroscopy for the placement of the prosthetic valve and is thus typically performed in the catheterization laboratory or hybrid operating room.
- Possible approaches for TAVR/TAVI include (Figure 1):
- Transfemoral
- Transaxillary/Transsubclavian
- Transcarotid
- Transaortic
- Transseptal
- Transapical
- Transcaval
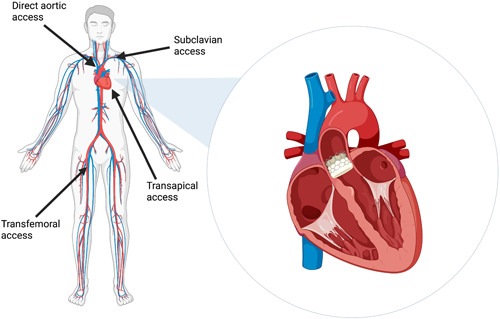
Figure 1. Common approaches for TAVR. Source: Srinivasan A, et al. Clin Cardiol. 2024;47(1): 24209. CC BY 4.0. PubMed
- Procedural stages
- Vascular access (venous and arterial sheath access is required.)
- Surgical cutdown vs percutaneous access
- Two sites of arterial access are usually required.
- Deployment of device
- Aortic/aortic root angiography
- Venous access is required for deployment of transvenous right ventricular pacing catheter.
- Placement of pacing wire into the right ventricle
- Determination of native aortic valve position
- Insertion of wire through the aortic valve
- Balloon aortic valvuloplasty
- Insertion of prosthetic valve sheath and positioning
- Rapid ventricular pacing (~180 bpm) to decrease cardiac output temporarily
- Prosthetic valve deployment
- Confirmation of appropriate deployment with minimal aortic valve regurgitation, lack of coronary ostial obstruction (e.g. fluoroscopy, TTE, or TEE)
- Sheath removal and vascular closure
- Valve types
- Balloon expandable
- Example: Edwards SAPIEN 3
- Self-Expanding
- Example: Medtronic CoreValve/Evolut
- Higher risk of high-grade heart block requiring pacemaker implantation
- Higher risk of paravalvular leak
Anesthetic Management
- When TAVR was first introduced, candidates were high-risk patients with multiple comorbidities. However, now even low-risk patients are being considered for TAVR.
- Hemodynamic considerations for the management of patients with AS undergoing TAVR include:
- Preload: maintain
- Afterload: maintain high to preserve coronary perfusion
- Contractility: maintain
- Heart rate: low to normal (60-80 bpm)
- Rhythm: normal sinus rhythm as much as possible
- Patients with AS tolerate loss of atrial contraction (e.g. atrial fibrillation) very poorly.
- Procedural considerations
- Monitored anesthesia care (MAC) is the most common modality used for transfemoral TAVR.
- Patients who are not candidates for MAC include, but are not limited to, those with poor ejection fraction requiring mechanical circulatory support during the procedure, severe OSA with propensity for airway obstruction, or significant acid reflux and concern for aspiration.
- General anesthesia is used for nontransfemoral approaches, when transesophageal echocardiography is needed, and for patients who are not candidates for MAC.
- Vascular access
- An arterial line and central line are commonly inserted.
- In many cases, (especially cases under MAC), may use lines placed by the cardiologist (ask for a larger sheath for venous access to piggy-back line).
- Preparation for potential large vascular injury and need for cardiopulmonary bypass
- Heparin will be required for the placement of arterial sheaths.
- During deployment of the prosthetic valve, clinicians should hold ventilation and perform rapid ventricular pacing to decrease the risk of malpositioning.
- Increases in blood pressure after deployment of TAVR are common.
- Monitored anesthesia care (MAC) is the most common modality used for transfemoral TAVR.
Complications
- Injury (dissection, perforation, occlusion) to vasculature traversed during the procedure (e.g., femoral artery, descending aorta, etc.) during both insertion and removal of sheaths
- Can cause retroperitoneal bleeding
- Aortic annular dissection or rupture
- Pericardial effusion/cardiac tamponade2
- Due to perforation of the ventricle, annular tear, aortic root rupture, etc.
- Can be recognized by TEE, TTE, or extravasation of contrast into the pericardium
- A high index of suspicion is required, as mortality for this complication is relatively high (23.5% in one study).
- While pericardiocentesis can be attempted to improve hemodynamics, this is often insufficient for definitive management and typically requires surgical correction.
- Misplacement/migration of deployed TAVR valve
- Occlusion of coronary ostia3
- Risk factors: female sex, larger TAVR valve sizes, valve-in-valve procedure, low-riding coronary ostia, shallow sinuses of Valsalva, elongated leaflets, significant calcified nodules
- Left-main coronary artery occlusion is more common than right coronary artery occlusion (incidence of 88% vs. 6.8%).
- There should be a high index of suspicion if ST changes and hypotension occur immediately following deployment.
- Management
- Hemodynamically, it is important to attempt perfusion via collaterals while balancing the effect of increased afterload on possibly failing ventricles. Also, myocardial oxygen demand (low-normal heart rate) should be reduced.
- The goal is to restore circulation as quickly as possible.
- May require mechanical circulatory support (e.g. VA-ECMO)
- The interventional proceduralist may be able to snare the valve and replace and/or manage with percutaneous coronary intervention.
- Often requires conversion to open surgery with cardiopulmonary bypass to restore perfusion
- High-grade/complete heart block requiring pacemaker support4,5
- Implantation of the valve can injure the AV node (causing complete heart block) or bundle branches (causing left bundle branch block).
- Presents as new onset block immediately after deployment when rapid pacing ends
- Transfemoral right ventricular pacing lead (previously used for rapid pacing) may be used for temporary pacing or a new temporary lead placed via the right internal jugular (IJ) vein. After placement of IJ pacemaker, ensure capture and adequate function prior to removal of the femoral pacemaker.
- Will require ICU monitoring after temporary pacemaker is placed
- Conduction disturbances may present up to 72 hours after procedure.
- Conduction disturbances may resolve; however, some patients may require permanent pacemaker placement. The incidence of permanent pacemaker insertion is higher for the Medtronic CoreValve (23.4-39%) compared to the Edwards Sapien valve (4.9-6%).
- Transient ischemic attack/embolic stroke6
- This should be in the differential for patients with altered mental status after TAVR, as embolic phenomena are almost universal.
- Early stroke (within 10 days of procedure) is primarily due to embolization of debris during approach and deployment.
- Late stroke (11 days to 1 year after TAVR) is associated with small body surface area, severe aortic calcification, and falls within the previous 6 months.
- However, the incidence of cerebrovascular injury is not significantly different in TAVR compared to SAVR.
- Paravalvular leak/aortic regurgitation
- Mitral valve impingement leading to mitral stenosis
References
- Brecker S. Transcatheter aortic valve implantation: Periprocedural and postprocedural management. In: Post T, ed. UpToDate; 2022. Updated July 11, 2023. Accessed July 26, 2024. Link
- Rezq A, Basavarajaiah S, Latib A, et al. Incidence, management, and outcomes of cardiac tamponade during transcatheter aortic valve implantation: a single-center study. JACC Cardiovasc Interv. 2012;5(12):1264-72. PubMed
- Sultan I, Siki M, Wallen T, Szeto W, Vallabhajosyula P. Management of coronary obstruction following transcatheter aortic valve replacement. J Card Surg. 2017 Dec;32(12):777-781. doi: 10.1111/jocs.13252. Epub 2017 Nov 16. PMID: 29143378. PubMed
- Young Lee M, Chilakamarri Yeshwant S, Chava S, Lawrence Lustgarten D. Mechanisms of Heart Block after Transcatheter Aortic Valve Replacement - Cardiac Anatomy, Clinical Predictors and Mechanical Factors that Contribute to Permanent Pacemaker Implantation. Arrhythm Electrophysiol Rev. 2015 Aug;4(2):81-5. doi: 10.15420/aer.2015.04.02.81. PMID: 26835105; PMCID: PMC4711557. PubMed
- Klein AA, Skubas NJ, Ender J. Controversies and complications in the perioperative management of transcatheter aortic valve replacement. Anesth Analg. 2014 Oct;119(4):784-798. doi: 10.1213/ANE.0000000000000400. PMID: 25232691. PubMed
- Davlouros PA, Mplani VC, Koniari I, Tsigkas G, Hahalis G. Transcatheter aortic valve replacement and stroke: a comprehensive review. J Geriatr Cardiol. 2018 Jan;15(1):95-104. doi: 10.11909/j.issn.1671-5411.2018.01.008. PMID: 29434631; PMCID: PMC5803543. PubMed
Other References
- Huffmyer J. TAVR. TEE Case of the Month January 2020. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.