Copy link
Study Design: Interventional Studies
Last updated: 08/21/2024
Key Points
- Randomized control trials (RCTs) are the most reliable method for determining causality in clinical research. They have diverse designs, such as active and placebo control trials, multiple-arm trials, cluster randomized trials, and adaptive designs.
- RCTs use various randomization methods such as simple, block, stratified, and covariate adaptive techniques to prevent biases and ensure accurate results.
- Blinding in clinical trials prevents biases by concealing group assignments from participants, clinicians, data collectors, and analysts.
Randomized Studies
- RCTs are the gold standard for validating the effectiveness and safety of interventions.1
- Participants are assigned to either a treatment or control group to isolate the intervention’s effect.1
- RCT designs include active control trials (superiority, equivalence, noninferiority), placebo controls, multiple-arm trials, cluster randomized trials, and adaptive designs.1
Control Arm Variations in RCTs
- In RCTs, the control arm can be an active control (established treatment) or placebo (inactive substance, sham treatment) (Figure 1).1
- Active control trials can be superiority, equivalence, or noninferiority studies, each comparing the experimental treatment to the standard of care (Figure 1).1
- Superiority study: Designed to determine if one intervention is significantly better than another.1
- Equivalence study: Designed to show two interventions have similar effects.1
- Noninferiority study: Designed to show that a new intervention is not worse than an existing treatment.1
- Placebo can be used to control for the placebo effect and reduce bias, with placebo studies aiming to determine whether the experimental treatment is superior to the placebo.1
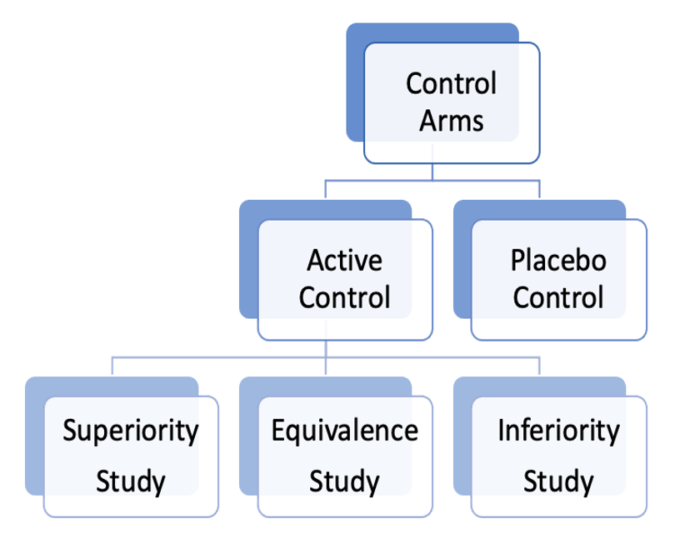
Figure 1. Control arm variations in RCTs
Multiple Arm Trials
- Multiple-arm trials fall into two categories:
- Dose-response studies: Tests different doses/regimens of an intervention against a control group1
- Comparative studies: Compare a single treatment group against multiple controls1
Cluster Randomized Trials (CRTs)
- CRTs randomize groups rather than individual participants.1
- They are often used in public health and educational research.1
- A notable variant is the stepped wedge design, where clusters receive the intervention in stages to manage logistics.1
Adaptive Designs
- Adaptive trial designs allow for modifications based on interim results, such as adjusting allocations if something proves more effective.1
- This flexibility helps address ethical concerns by potentially stopping less effective treatments and improving outcomes.1
- Adaptations and their statistical criteria must be planned a priori.1
Randomization
- The randomization process in RCTs helps to prevent biases and ensure accurate results by minimizing group differences.1 Various randomization methods exist, including simple, block, stratified, and adaptive techniques.2
Simple Randomization
- Simple randomization assigns participants using a single random sequence, ensuring complete randomness.2
- Common methods include coin flips, dice rolls, or random number generators.2
- This process is easy to implement and results in balanced groups in large trials but can cause unequal groups in smaller trials.2,3
Block Randomization
- Block randomization ensures equal sample sizes by assigning participants in small, predetermined blocks (Figure 2).2
- Randomization occurs within each block to ensure a balanced allocation of participants to different groups.3
- Block sizes (e.g., 4, 6, 8) should be multiples of the number of groups (Figure 2).2
- This method maintains balanced group sizes but may not ensure comparability of certain covariates.2,3
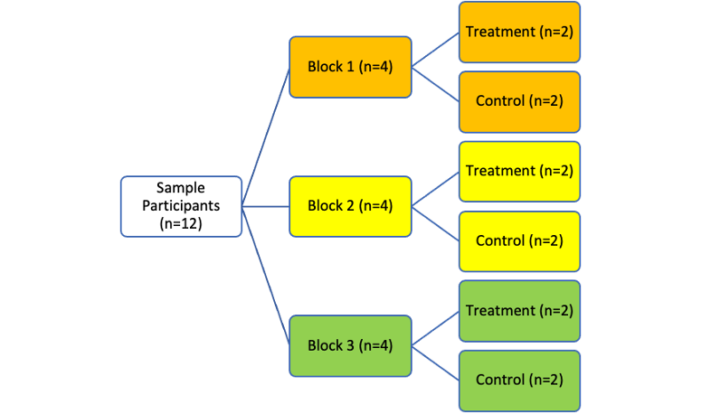
Figure 2. Process of block randomization in RCTs
Stratified Randomization
- Stratified randomization ensures balanced groups by accounting for covariates.2
- In this method, covariates are identified, and participants are assigned to blocks based on these covariates, with randomization occurring within blocks.2
- This method reduces confounding variables, ensuring that differences are due to the treatment rather than covariate imbalances.4
- It also allows for meaningful subgroup analyses and more confident interim analyses by maintaining group balance.4
- Stratification in interim analyses can improve the validity and power of the study, making it easier for clinicians to detect treatment differences and adverse events, especially when faced with small sample sizes.4
Covariate Adaptive Randomization
- Covariate adaptive randomization prevents imbalances in significant covariates among treatment groups.2
- It balances vital characteristics by continuously adjusting group assignments during the trial.2
- A concern with this technique is that it can lead to predictable treatment assignments, which can undermine the randomization process.2
- To mitigate this, experts suggest randomly assigning a small number of participants before applying covariate adaptive randomization.2
- The complex nature of covariate adaptive randomization increases the administrative burden, limiting its practical use.2
Blinding
- Blinding involves hiding which groups the participants belong to from both the participants and researchers.5 This helps prevent biases in treatment administration and how outcomes are assessed/reported.5
- Blinding is commonly implemented in RCTs to ensure that observed outcome differences are attributed to the intervention rather than behavior that may be influenced by knowledge of group assignments.5
Blinding in Clinical Trials
- Blinding is flexible, allowing investigators to create novel techniques as long as they effectively conceal group information.5,6
- Researchers should specify who was blinded and how it was done and verify its effectiveness.5
- Blinding should involve participants, clinicians, data collectors, and data analysts (Figure 3).5
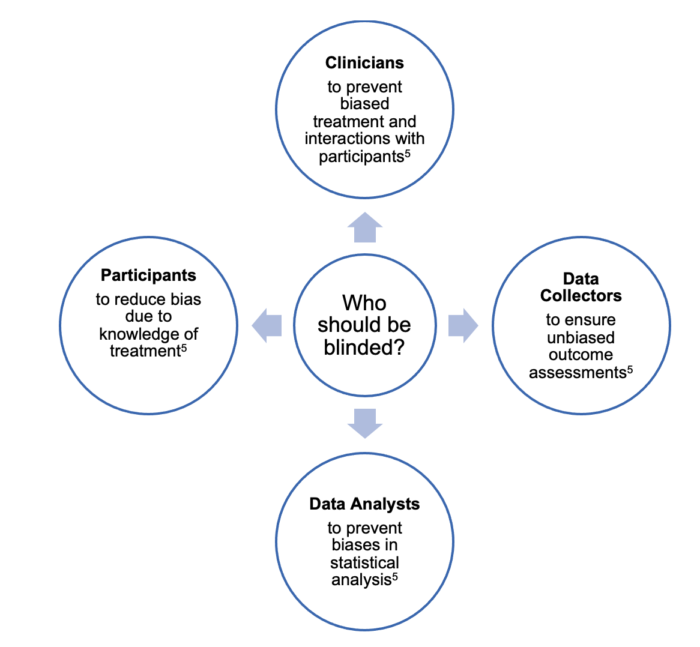
Figure 3. Who should be blinded in a randomized control trial?
Blinding Techniques and Methods in Clinical Trials
- Standard blinding methods include utilizing identical treatments or masking treatment characteristics (e.g., opaque covering).7
- Blinding of participants and providers is commonly achieved through centralized preparation of identical tablets, bottles, and syringes.6
- Flavoring can also be used to mask the taste of active oral treatments.6
- The double-dummy technique is used when comparing two treatments that are not identical and involves using two sets of placebos, one for each treatment, to ensure successful blinding.6,7
- Blinding participants in surgical/procedure trials is achievable through strategies like imitating surgical access points, replicating operating room cues, matching the duration of procedures, and standardization of care.6
- Methods to prevent unblinding include employing active placebos, centralizing both side effect assessments and dosage adjustments, and providing partial information to patients regarding side effects.7
References
- Zabor EC, Kaizer AM, Hobbs BP. Randomized controlled trials. Chest. 2020;158(1S):S79-S87. PubMed
- Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43(2):215-21. PubMed
- Efird J. Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health. 2011;8(1):15-20. PubMed
- Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clinical Epidemiol 1999;52(1):19-26. PubMed
- Karanicolas PJ, Farrokhyar F, Bhandari M. Practical tips for surgical research: blinding: who, what, when, why, how? Can J Surg. 2010;53(5):345-8. PubMed
- Monaghan TF, Agudelo CW, Rahman SN, et al. Blinding in clinical trials: Seeing the big picture. Medicina. 2021;57(7):647. PubMed
- Boutron I, Estellat C, Guittet L, et al. Methods of blinding in reports of randomized controlled trials assessing pharmacologic treatments: a systematic review. PLoS Med. 2006;3(10):e425. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.