Copy link
Root Cause Analysis
Last updated: 08/02/2024
Key Points
- A root cause analysis (RCA) is a structured process by which a group or organization examines an event or outcome by analyzing specific potential causes to mitigate these potential outcomes in the future.
- An RCA utilizes a specific group of individuals within the organization to analyze the outcome and design appropriate interventions when necessary.
- The reasons for conducting an RCA may stem from an event classified by the severity of harm and a risk profile based on the concern surrounding that particular event. Additionally, regulatory bodies (such as the Joint Commission, etc.) uphold standards of practice and safety and may necessitate an RCA if an event occurs.
Introduction
- According to the Institute of Medicine, quality goals in the perioperative arena may include, but are not limited to, “efficient, safe, reliable, effective, and equitable” care.1
- These quality goals may be met by conducting an RCA with the following objectives:
- Addressing ongoing safety issues as they arise in an organization (e.g., a sentinel event)
- Meeting criteria set by regulatory bodies
- Aiming to achieve a national benchmark that is set by a regulatory body or other organization
- An RCA is a method that utilizes a team to determine the root cause of a specific unanticipated event within a system.
- The RCA tool is grounded in Safety-1, which focuses on Safety Analytics and Quality and seeks to identify and define the discussed problem.
- The safety leadership in a department or hospital may initiate an RCA as a response to a sentinel event or an event considered to have caused patient harm or potential patient harm.
- Once the “failure” or root cause is identified, the team may implement a quality improvement project to improve care processes, technology, facilities, and communications.
Safety Event Classification
- Regulatory bodies create and uphold specific standards for practice and safety in hospitals and perioperative settings. Depending on the type of event, their standards or requirements may push an organization to conduct an RCA.2 Regulatory bodies may play a role in accreditation, compliance, and safety to help generate appropriate benchmarking metrics for hospitals.
- Classifications of severity of safety events based on outcomes can be categorized by Sentinel Event, Serious Safety Event, Precursor Safety Event, and Near Miss Event (Table 1).
- Many organizations utilize the Safety Event Classification decision tree (Figure 1) to categorize Patient Safety events.2 The Safety Event Classification systems answer the following questions to make their determinations:
- Did the event reach the patient?
- Did the event cause harm? (Table 2)
- A hospital may determine that a further investigation or RCA is appropriate based on the classification of the event and the outcome.
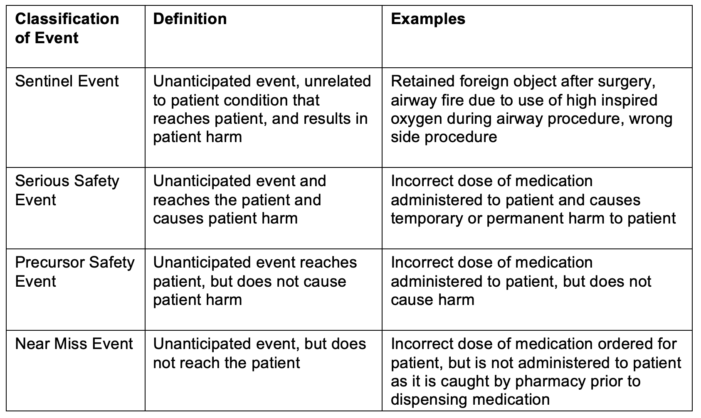
Table 1. Classifications of patient harm
Adapted from Troop C, Stockmeier C: SEC & SSER Patient Safety Measurement System for Healthcare. HPI White Paper Series 2011. Link
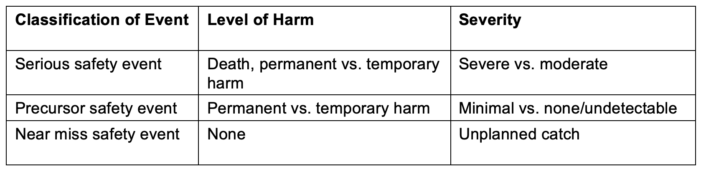
Table 2. Definitions of harm
Adapted from Troop C, Stockmeier C: SEC & SSER Patient Safety Measurement System for Healthcare. HPI White Paper Series 2011. Link
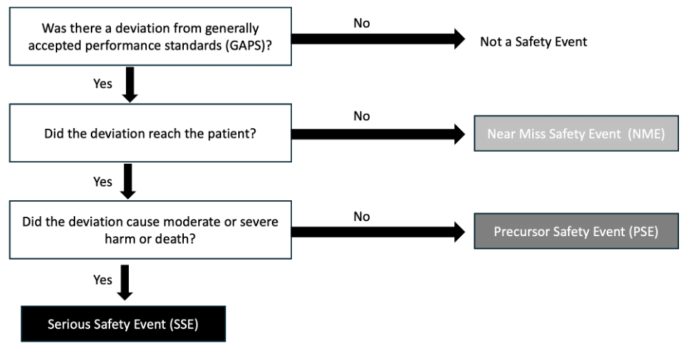
Figure 1. Healthcare Performance Improvement (HPI) taxonomy of safety event classification
Adapted from Troop C, Stockmeier C: SEC & SSER Patient Safety Measurement System for Healthcare. HPI white paper. Link
RCA Process
- RCAs start by identifying a “component failure” leading to the event and then trace the process from start to finish as the various factors are discussed.
- Both individual and system factors are discussed as potential causes.
- Proximate causes and contributing causes are examined to determine if changes in current processes are necessary to improve patient safety.
- Sometimes, QI methods are deployed to attempt to “fix” the system’s failure after it occurs.3
- The process may include 2-4 meetings for planning and execution of interventions, with a longer-term follow-up meeting after action items are completed.
- The discovery meeting is an opportunity to hear directly from team members involved in the event and disclose specific details about it.
- Team members are often able to provide insight into the circumstances or human factors that affected the sequence of events that occurred.
- In addition to the team members involved in the case, safety officers from the appropriate departments, an executive or physician leader for the RCA may provide value and insight into the system’s processes and guidance for discussing adapting processes.
- If the RCA team determines that changes or processes will require adaptation, utilizing action items with responsible team members or a Gantt Chart (a timeline of an expected process to change) may be helpful.
Shortcomings of the RCA Process
- The shortcomings of RCA processes are essential to discuss as the RCA discussion tends to focus on a single event as it occurs.
- Chances of a similar event occurring in the same way may be minimal.
- It is essential to discuss high-reliability principles here as they are typically more fitting for complex systems and more consistently seen with challenging patients in our perioperative world. How can we make it easier to do the right thing? For example, if we care for patients of vastly different weights in a fast-paced surgery center, necessitating different medication dosages, it might be anticipated that stocking various concentrations of the same medication could lead to a potential medication error (morphine 0.1 mg/ml versus morphine 10 mg/ml).
- While RCAs are supposed to be focused on the team and the potential for systems failures, many people believe that RCAs are more blame and fault-finding missions, though they claim to be independent of these.
- The “Swiss-Cheese model” is a theory that focuses on latent failures, or failures that occur as “holes” within multiple systems that allow for an event or error to occur.
- This model is a straightforward way of dissecting a problem. In reality, our current system is much more complex.
- In a system that does not deal with complex patients and volatile conditions, a problem may have a “start” and a “finish” (i.e., a cause and event).
- For an environment to function, many complex scenarios and volatile conditions must be uncertain on a daily basis.
- Human factors are often analyzed in an RCA and will discuss causes classified by organizational influences, supervisory factors, and preconditions for unsafe and unsafe acts.
- These can be further broken down into the following areas: organizational culture, operational process, resource management, inadequate supervision, planned inappropriate operations, failure to correct a known problem and supervisory violations.4
- While some teams utilize these basic concepts, others seek to utilize “safety differently” concepts to minimize blame and better understand processes. Understanding human factors and processes around managing unanticipated events is crucial to initiating changes and discussing organizational resilience, which is seen more from a Safety-II perspective.
Example of an RCA Process
- Buck D et al. discuss an example of an RCA process for an infant who developed local anesthetic toxicity from an overdose of bupivacaine (Figure 2).5
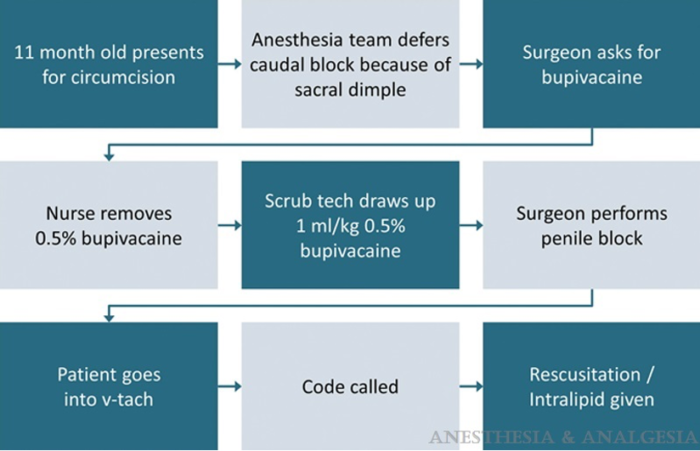
Figure 2. Example of a sequence of events for an RCA: 11-month-old receiving an overdose of bupivacaine
Used with permission from Buck D. et al. Case discussion and root cause analysis. Anesth Analg. 2014;119(1); 137–40. PubMed
- An analysis of the proximate cause, contributing factors, and preventive actions are listed in Table 3.5

Table 3. An example of the proximate cause, contributing factors, and preventive actions in an RCA5
References
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001. Link
- Troop C, Stockmeier C: SEC & SSER Patient Safety Measurement System for Healthcare. HPI White Paper Series 2011;1-7. Link
- Wilson PF, Dell LD, Anderson GF. (1993). Root cause analysis: A tool for total quality management. Milwaukee, Wisconsin: ASQ Quality Press.
- Wiegmann DA, Wood LJ, Cohen TN, et al. Understanding the “Swiss Cheese Model” and Its Application to Patient Safety. J Patient Saf. 2022;18(2): 119–23. PubMed
- Buck D, Kreeger R, Spaeth J. Case discussion and root cause analysis. Anesthesia and Analgesia. 2014;119(1); 137–140. PubMed
Other References
- Gaba DM. Special Issue: Safety first: Ensuring quality care in the intensely productive environment - The HRO model. Anesthesia Patient Safety Foundation. Link
- Beecher HK, Todd DP. A study of deaths associated with anesthesia and surgery. Based on a study of 559,548 anesthesias in ten institutions 1948-1952. Ann Surg. 1954; 140:2-35. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.