Copy link
Renin-Angiotensin-Aldosterone System
Last updated: 04/14/2025
Key Points
- The renin-angiotensin-aldosterone system (RAAS) is a critical regulator of blood pressure, fluid balance, and vascular tone.
- Overactivation of RAAS can lead to conditions like hypertension, heart failure, and kidney disease.
- If patients are on RAAS inhibitors (angiotensin-converting enzyme [ACE] inhibitors, angiotensin II receptor blockers [ARBs]), decisions should be made on whether to continue or discontinue them preoperatively based on the risk of hypotension.
- Fluctuations in RAAS activation may occur during different anesthetic stages (induction, maintenance, emergence), increasing the risk of hemodynamic instability in patients with RAAS dysregulation.
Introduction
- The RAAS is a hormone-based pathway that plays a critical role in regulating blood pressure, fluid levels, and electrolyte balance. This system is essential for regulating renal, cardiac, and vascular physiology, but it can also contribute to serious health issues like hypertension, heart failure, and renal disease if it becomes overactive.1 Because of this, RAAS is a common target for medications used to treat cardiovascular and kidney diseases.2
- Heart failure and kidney disease are prevalent comorbidities in surgical patients, with nearly one-third of adults undergoing noncardiac procedures and two-thirds of those undergoing coronary revascularization reporting a history of hypertension.3 Given the high prevalence of these conditions, it is crucial to consider their impact during anesthesia management, as they can significantly influence perioperative outcomes and require careful monitoring and adjustment of anesthetic techniques.
Overview of RAAS
- The RAAS involves a tightly regulated sequence of hormonal interactions designed to maintain blood pressure and fluid balance. When blood flow to the kidneys decreases – due to dehydration, blood loss, or hypotension – the juxtaglomerular cells in the kidneys release renin into the bloodstream.1
- Renin then acts on angiotensinogen, a protein produced by the liver, converting it into angiotensin I. This inactive form is quickly transformed into angiotensin II by angiotensin-converting enzyme (ACE), primarily located in the lungs.
- Angiotensin II stimulates the adrenal cortex to release aldosterone from the zona glomerulosa, the posterior lobe of the hypothalamus, to secrete vasopressin (ADH) and is a potent arteriolar vasoconstrictor. Aldosterone also acts on the kidneys to promote sodium and water reabsorption and potassium excretion, thereby increasing blood volume and pressure. In a healthy state, this system self-regulates through negative feedback.4
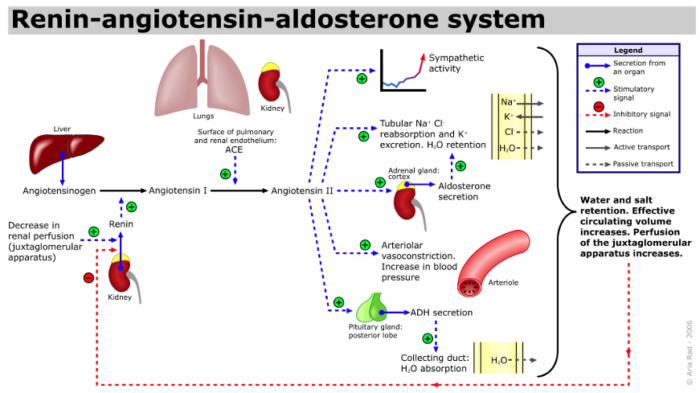
Figure 1. Renin-angiotensin-aldosterone system. Source: A. Rad(me), Wikimedia Commons. CC BY SA 3.0. CC BY-SA 3.0
- In addition to the classical view of the endocrine RAAS system, there are several local renin-angiotensin systems at the tissue level that function independently of each other with autocrine (cell-to-same cell) and paracrine (cell-to-different cell) effects.1
- In pathological conditions, such as chronic kidney disease, congestive heart failure, or primary hyperaldosteronism, the RAAS can become overactivated, leading to persistent vasoconstriction, fluid retention, and hypertension, which further strain the cardiovascular system and worsen disease progression.1
Pharmacological Management of RAAS Overactivity
- The blockade of RAAS at several points along its pathway has represented an innovative development in the prevention and treatment of cardiac and renal diseases1 (Figure 2).
- ACE inhibitors and ARBs are commonly used as first- or second-line agents in the treatment of hypertension.2,4
- As the name suggests, ACE inhibitors inhibit the enzyme ACE, resulting in decreased formation of angiotensin II (vasodilation and decreased blood pressure) and increased bradykinin activity. ARBs, on the other hand, impair the binding of angiotensin II to the AT1 receptor on the cell membrane, inhibiting the action of angiotensin II (decreased blood pressure).2,4
- The antihypertensive effects of ACE inhibitors and ARBs are enhanced by the concomitant administration of a low dose of diuretics.2
- Aliskiren is a direct renin inhibitor that also lowers blood pressure. However, it is not commonly used.2
- Angiotensin receptor-neprilysin inhibitors combine an ARB with a neutral endopeptidase inhibitor (for example, valsartan and sacubitril) that increase the levels of natriuretic peptides.4
- Nonsteroidal mineralocorticoid receptor antagonists are a new class of medications that cause a conformational change of the receptor that affects the recruitment of transcriptional coactivators, leading to decreased activation of proinflammatory molecules.4
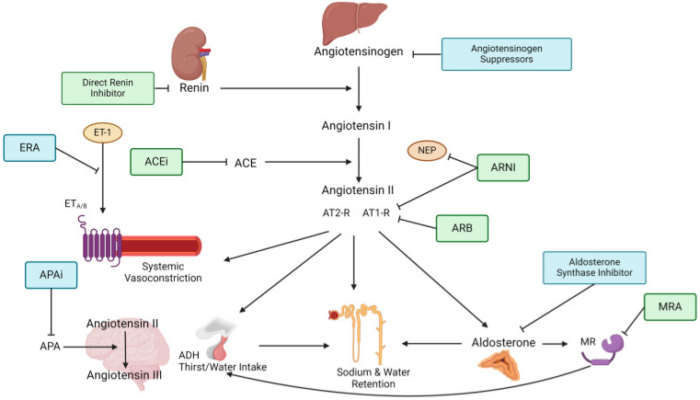
Figure 2. Drug Inhibition of RAAS cascade.
(ACE = Angiotensin-converting enzyme, ACEi = Angiotensin-converting enzyme inhibitor, ADH = Anti-diuretic hormone, APA = Aminopeptidase A, APAi = Aminopeptidase A inhibitor, ARB = Angiotensin II type 1-receptor-blocker, ARNI = Angiotensin II type 1-receptor-neprilysin-inhibitor, AT1-R = Angiotensin type 1-receptor, AT2-R = Angiotensin type 2-receptor, ERA = Endothelin-receptor antagonist, ET-1 = Endothelin-1, ETA/B = Endothelin-receptor type A or B, MR = Mineralocorticoid receptor, MRA = Mineralocorticoid-receptor antagonist, NEP = neutral peptidase). Source: Ksiazek SH, et al. Renin-angiotensin-aldosterone system: From history to practice of a secular topic. Int J Mol Sci. 2024. CC-BY 4.0.
Perioperative Antihypertensive Medication Management
- The decision to continue or withhold ACE inhibitors and ARBs before surgery remains debated. These medications may interfere with the body’s compensatory ability to regulate blood pressure during surgical procedures, potentially leading to extended periods of low blood pressure.5
- Continuing ACE inhibitors or ARBs before surgery has been linked to a greater risk of both intraoperative and postoperative hypotension in several studies. However, not all research supports this finding consistently.
- In the Stop-or-Not trial involving 2,222 patients treated with renin-angiotensin system inhibitors (RASIs) for a minimum of three months prior to undergoing major noncardiac surgery, a higher incidence of intraoperative hypotension—defined as a mean arterial pressure below 60 mmHg or the requirement for vasopressor support—was observed in the group that continued RASI therapy compared to the group that discontinued treatment (54% vs. 41%; relative risk 1.31, 95% CI 1.19–1.44).6 However, comparable rates of perioperative hypotension have been reported in other studies.5
- In contrast, stopping these medications before surgery has been associated with an increased risk of elevated blood pressure after the operation. In a randomized study involving 275 patients, a greater incidence of hypertensive events—defined as systolic blood pressure exceeding 180 mmHg—was observed among those assigned to discontinue ACE inhibitor therapy, compared to those who continued treatment (24% vs. 12%; relative risk 1.95, 95% CI 1.14–3.34).7
- No significant differences in cardiovascular or respiratory outcomes, postoperative mortality, or major complications have been observed between the continuation and discontinuation of ACE inhibitors or ARBs in the perioperative period.5
- The choice to either continue or stop ACE inhibitors and ARBs is determined by the medication’s indication, the patient’s blood pressure status, and the planned surgical procedure and anesthesia. If there is a significant risk of perioperative hypotension, these drugs are typically withheld on the morning of surgery. However, when the drugs are indicated for conditions such as heart failure or poorly controlled hypertension, they are generally continued to prevent the worsening of the underlying condition.
- Failure to restart ACE inhibitors or ARBs within 48 hours of surgery has been associated with higher 30-day mortality rates in observational studies.5
References
- Fisher ND. Overview of the renin-angiotensin system. In: Post T, ed. UpToDate; September 27, 2023. Accessed April 12, 2025. Link
- Mann JF, Hilgers KF. Renin-angiotensin system inhibition in the treatment of hypertension. In: Post T, ed. UpToDate: May 30, 2023. Accessed April 12, 2025. Link
- Schonberger RB, Fontes ML, Selzer A. Anesthesia for Patients with Hypertension. In: Post T, ed. UpToDate: October 2, 2023. Accessed April 12, 2025. Link
- Ksiazek SH, Hu L, Andò S, et al. Renin–Angiotensin–Aldosterone System: From history to practice of a secular topic. Int J Mol Sci. 2024; 25(7):4035. PubMed
- Muluk V, Cohn SL, Whinney C. Perioperative medication management. In: Post T, ed. UpToDate; November 6, 2024. Accessed April 12, 2025. Link
- Legrand M, Falcone J, Cholley B, et al. Continuation vs. discontinuation of renin-angiotensin system inhibitors before major noncardiac surgery: The Stop-or-not randomized clinical trial. JAMA. 2024;332(12): 970. PubMed
- Ackland GL, Patel A, Abbott TEF, et al. Discontinuation vs. continuation of renin-angiotensin system inhibitors before non-cardiac surgery: The SPACE trial. Eur Heart J. 2024:45(13): 1146. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.