Copy link
Regional Anesthesia in Patients Receiving Antithrombotic or Thrombolytic Therapy: Part 2
Last updated: 04/06/2023
Key Points
- For these recommendations, the American Society of Regional Anesthesia and Pain Medicine (ASRA) incorporated FDA-approved labeling and a pharmacologic approach to newer anticoagulants where data are sparse. Recommendations with time intervals reflect normal variation in pharmacological activity, and caution must be taken with patients who have comorbidities that may affect metabolism, excretion, and/or distribution.1
- ASRA recommends against the performance of neuraxial techniques in patients receiving parenteral thrombin inhibitors (e.g., desirudin, bivalirudin, argatroban) (grade 2C).
- Nonsteroidal anti-inflammatory drugs (NSAIDs) appear to represent no added significant risk of the development of spinal hematoma in patients undergoing neuraxial techniques. However, in patients receiving NSAIDs, ASRA suggests caution in the performance of neuraxial techniques if the concurrent use of medications affecting clotting mechanisms is anticipated in the early postoperative period due to an increased risk of bleeding complications.
Introduction
- In 2018, ASRA partnered with National Partnership for Maternal Safety (NPMS), and the Society for Obstetric Anesthesia and Perinatology (SOAP) to create the 4th edition of the ASRA guidelines on regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy.1,2
- Notable updates from the 2015 guidelines include new and adjusted recommendations for newer medications, including herbal supplements and the routine use of physical exam findings for signs of coagulopathy.1,3
- It is important to note that these guidelines do not define the standard of care and are not intended to replace clinical judgment. The phrase “ASRA recommends” is used for strong recommendations (grades 1A, 1B, and 1C), and “ASRA suggests” is used for weaker recommendations (grades 2A, 2B, and 2C).1
- ASRA suggests that these guidelines may be applied to parturients (grade 2C). However, in circumstances involving select high-risk parturients receiving venous thromboembolism (VTE) prophylaxis and requiring urgent interventions for maternal or fetal indications, exceptions/modifications of guidelines may be appropriate (grade 2C).
- This summary will focus on the recommendations for patients on antifactor Xa agents, direct thrombin inhibitors, warfarin, and herbal therapy.
Antifactor Xa Agents
- Fondaparinux: ASRA suggests the removal of neuraxial catheters 6 hours prior to the first postoperative dose (grade 2C).1
- Rivaroxaban: ASRA suggests that rivaroxaban be discontinued 72 hours prior to a neuraxial block (grade 2C). ASRA suggests removing neuraxial catheters 6 hours prior to the first postoperative dose (grade 2C). With unanticipated administration with an indwelling catheter, ASRA suggests holding rivaroxaban dosing for 22-26 hours before or collecting anti-factor Xa assay calibrated to rivaroxaban prior to catheter removal (grade 2C).
- Apixaban: ASRA suggests that apixaban be discontinued 72 hours prior to a neuraxial block (grade 2C). ASRA suggests removing neuraxial catheters 6 hours prior to the first postoperative dose (grade 2C). With unanticipated administration with an indwelling catheter, ASRA suggests holding apixaban for 26-30 hours or collecting anti-factor Xa assay calibrated to apixaban prior to catheter removal (grade 2C).
- Edoxaban: ASRA suggests that edoxaban be discontinued 72 hours prior to a neuraxial block (grade 2C). ASRA suggests removing neuraxial catheters 6 hours prior to the first postoperative dose (grade 2C). With unanticipated administration with an indwelling catheter, ASRA suggests holding apixaban for 20-28 hours or collecting anti-factor Xa assay calibrated to edoxaban prior to catheter removal (grade 2C).
- Betrixaban: ASRA suggests that betrixaban be discontinued 72 hours prior to a neuraxial block (grade 2C). ASRA suggests removing neuraxial catheters 5 hours prior to the next dose (grade 2C). With unanticipated administration with an indwelling catheter, ASRA suggests holding betrixaban dosing for 72 hours before catheter removal (grade 2C).
Direct Thrombin Inhibitors
Parenteral Thrombin Inhibitors
- In patients receiving parenteral thrombin inhibitors (e.g., desirudin, bivalirudin, argatroban), ASRA recommends against the performance of neuraxial techniques (grade 2C).
Oral Thrombin Inhibitors
- In patients receiving oral thrombin inhibitors (e.g., dabigatran), ASRA suggests that dabigatran be discontinued 120 hours prior to a neuraxial block. However, if renal function has been reliably determined, and there are no additional risk factors for bleeding (e.g., age >65 years, hypertension, concomitant antiplatelet medications), a more graded approach may be considered:
- Creatinine clearance (CrCl) 80mL/min or greater: ASRA suggests that dabigatran be discontinued 72 hours prior to neuraxial block. Consider checking dilute thrombin time (dTT) or ecarin clotting time (ECT) if less than 72 hours. An acceptable level of residual dabigatran activity to proceed with neuraxial block remains undetermined (grade 2C).
- CrCl 50 to 79 mL/min: ASRA suggests dabigatran be discontinued 96 hours prior to neuraxial block. Consider checking dTT or ecarin clotting time (ECT) if less than 96 hours. An acceptable level of residual dabigatran activity to proceed with neuraxial block remains undetermined (grade 2C).
- CrCl 30 to 49 mL/min: ASRA suggests dabigatran be discontinued 120 hours prior to neuraxial block. Consider checking dTT or ecarin clotting time (ECT) if less than 120 hours. An acceptable level of residual dabigatran activity to proceed with neuraxial block remains undetermined (grade 2C).
- CrCl less than 30 mL/min: ASRA suggests against the performance of neuraxial blocks (grade 2C).
- ASRA suggests that neuraxial catheters be removed 6 hours prior to the first postoperative dose of dabigatran (grade 2C). With unanticipated administration with an indwelling catheter, hold dabigatran for 34-36 hours or assess dTT or ECT prior to removal.
Warfarin
- Caution should be used when performing neuraxial techniques with recently discontinued chronic warfarin therapy. ASRA recommends warfarin must be stopped ideally 5 days prior to the planned procedure, and the international normalized ratio (INR) is normalized prior to the initiation of a neuraxial block (grade 1B).
- In patients receiving an initial dose of warfarin prior to surgery, ASRA suggests the INR be checked prior to neuraxial block if the first dose was given more than 24 hours earlier or a second dose of warfarin has been administered (grade 2C).
- ASRA suggests removing neuraxial catheters when the INR is less than 1.5. While the removal of epidural catheters 12 to 24 hours after warfarin administration does not appear to increase risk, there may be a higher risk of catheter removal after 48 hours of warfarin therapy (grade 2C).
- ASRA suggests continuing neurologic assessment for at least 24 hours following catheter removal (grade 2C).
- ASRA has no definitive recommendation for catheter management in patients with therapeutic levels of anticoagulation during neuraxial catheter infusion (grade 2C).
Antiplatelets
- NSAIDs without concurrent use of other medications affecting clotting mechanisms: ASRA does not identify specific concerns as to the timing of single-injection or catheter techniques in relationship to the dosing of NSAIDs, postoperative monitoring, or the timing of neuraxial catheter removal (grade 1A).
- Thienopyridines: ASRA recommends the following preoperative time interval between discontinuation of therapy and neuraxial blockade (grade 1C).
- Ticlopidine: 10 days.
- Clopidogrel: 5-7 days.
- Prasugrel: 7-10 days.
- ASRA suggests that neuraxial catheters should not be maintained with prasugrel or ticagrelor due to rapid onset, but as the antiplatelet effect is not immediate with ticlopidine and clopidogrel, neuraxial catheters may be maintained for 1-2 days provided a loading dose was not administered (grade 2C).
- Ticagrelor: ASRA recommends the preoperative time interval between discontinuation of ticagrelor is 5 to 7 days (grade 1C). Ticagrelor may be resumed immediately after catheter removal, provided a loading dose is not administered. If a loading dose is administered, ASRA suggests a time interval of 6 hours between removal and administration. Neuraxial catheters should not be maintained while on ticagrelor due to rapid onset (grade 2C).
- Platelet Glycoprotein (GP) IIb/IIIa inhibitors: Preoperatively, ASRA recommends that neuraxial techniques be avoided until platelet function – as impacted by the GP IIb/IIIa inhibitor – has recovered (patients are typically on dual therapy and may still have residual NSAID effect). Postoperatively, they are contraindicated within 4 weeks of surgery. If emergently administered postoperatively following neuraxial technique, the timing of catheter removal is based on the ongoing risk of thromboembolism, and need for continued antithrombotic therapy, and the potential for spinal bleeding during catheter maintenance and removal.
- Cilostazol: ASRA suggests that neuraxial techniques be avoided for 2 days after discontinuation of cilostazol (grade 2C). ASRA suggests removal of the catheter prior to the reinstitution of therapy postoperatively and that the first postoperative dose can be administered 6 hours after removal (grade 2C).
- Dipyridamole: ASRA suggests discontinuing extended-release dipyridamole for 24 hours prior to neuraxial block. Aspirin may be continued perioperatively (grade 2C). ASRA suggests removal of the catheter prior to the reinstitution of therapy postoperatively and that the first postoperative dose can be administered 6 hours after removal (grade 2C).
- Cangrelor: Based on the elimination half-life, ASRA suggests that neuraxial techniques be avoided for 3 hours after discontinuation of cangrelor (grade 2C). ASRA suggests removal of catheter prior to reinstitution of therapy postoperatively and that the first postoperative dose can be administered 8 hours after removal (grade 2C).
Herbal Therapy
- Herbals supplements with the greatest impact on hemostasis include garlic, ginkgo, and ginseng.
- ASRA recommends against the mandatory discontinuation of these medications or avoidance of regional anesthetic techniques in patients taking these medications (grade 1C).
See also: Regional Anesthesia in Patients Receiving Antithrombotic or Thrombolytic Therapy: Part 1
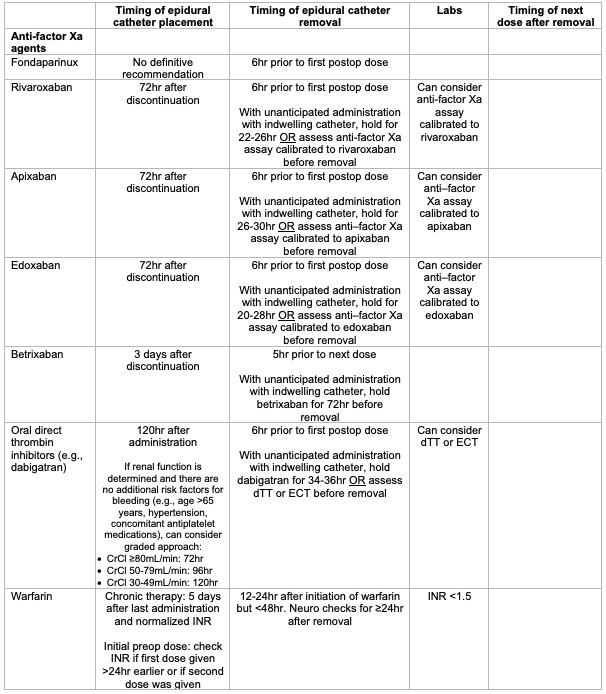
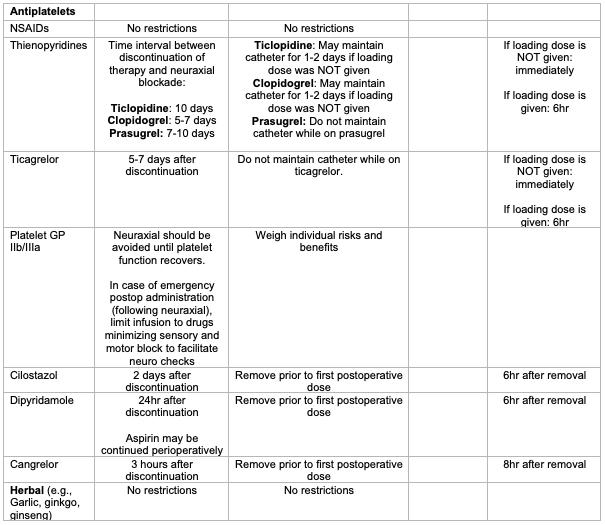
Table 1. Summary of ASRA recommendations for epidural catheter placement and removal in patients on anti-factor Xa agents, anti-platelet agents, and herbal supplements.
References
- Horlocker TT, Vandermeuelen E, Kopp SL, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (Fourth edition) Reg Anesth Pain Med. 2018;43(3):263-309. PubMed
- Leffert L, Butwick A, Carvalho B, et al. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126(3):928-944. PubMed
- Wickboldt A, Guirguis M. ASRA Pain Medicine News. ASRA Pain Medicine. Published August 27, 2019. Accessed October 30, 2022. Link
- Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (Fourth edition): Erratum. Reg Anesth Pain Med. 2018;43(5):566. PubMed
- Tryba M. European practice guidelines: thromboembolism prophylaxis and regional anesthesia. Reg Anesth Pain Med. 1998;23(6 Suppl 2):178-182. PubMed
Other References
- Bauer ME, Arendt K, Beilin Y, et al. The Society for Obstetric Anesthesia and Perinatology Interdisciplinary Consensus Statement on Neuraxial Procedures in Obstetric Patients With Thrombocytopenia. Anesth Analg . 2021 Jun 1;132(6):1531-1544. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.