Copy link
Perioperative Brain Health: Anesthesia Neurotoxicity
Last updated: 10/21/2024
Key Points
- Animal studies have shown that commonly used anesthetic agents, such as volatile agents, GABAA agonists, and NMDA antagonists, can induce neuroapoptosis and alter synaptogenesis. When administered during critical periods of brain development, these agents can lead to structural and functional changes in the brain and long-term cognitive and behavioral deficits.
- Human studies on anesthesia-induced neurotoxicity in the developing brain have provided mixed results due to difficulties in study design. While single, short-term exposures to anesthesia do not appear to result in significant cognitive deficits, there is concern about the potential effects on executive function or behavior and the impact of multiple or prolonged exposures to anesthetic agents.
- Continued investment in large-scale, longitudinal studies is essential to understanding the long-term effects of anesthesia on pediatric brain development. Research targets may include better determining the neurodevelopmental effects of anesthetic agents, identifying vulnerable patient populations, and developing strategies to mitigate neurotoxic effects.
Animal Data
- Timeline for preclinical (animal) evidence for anesthesia-induced neurotoxicity:
- The earliest evidence for anesthesia-induced neurotoxicity was published in 1999 when Ikonomidou et al. demonstrated that exposure to NMDA receptor antagonists induced widespread neuronal apoptosis in fetal and early neonatal rats.1
- Jevtovic-Todorvic et al. demonstrated the potential clinical relevance of this finding. They showed that exposure to other anesthetic agents, including isoflurane, nitrous oxide, and midazolam, was associated with neuroapoptosis and persistent learning and memory impairments in rats.1
- Subsequent preclinical studies indicated that exposure to all commonly used anesthetic agents can cause some degree of neuronal injury and neurobehavioral impairment across various animal models.1
- The transition to clinical (human) studies began in 2009 (see below).
- Summary of animal data:1
- The majority of animal studies have demonstrated persistent neuronal injury and neurobehavioral impairment after ante- or postnatal exposure to anesthetic agents. Early studies focused on deficits in learning and memory, while more recent studies have demonstrated behavioral changes, including delayed development, social withdrawal, and anxious, depressive, or hyperactive behaviors.
- Nearly all anesthetic drugs, including volatile agents, nitrous oxide, ketamine, propofol, benzodiazepines, barbiturates, etomidate, and dexmedetomidine, have been studied and associated with adverse outcomes.
- The effect of anesthetic agents is typically dose-dependent, with more neuronal injury and neurobehavioral impairment observed after longer or repeated exposures and higher doses of anesthetic agents.
- These effects have been seen across multiple animal models, including rats, mice, sheep, rabbits, guinea pigs, piglets, and nonhuman primates.
- Animal studies have also been used to investigate potential strategies to mitigate the neurotoxic effects of anesthesia, such as pharmacological interventions, environmental enrichment, and anesthesia protocols that minimize exposure or utilize alternative agents with potentially lower neurotoxicity.1
- Limitations of animal studies:
- Preclinical data have been criticized for several reasons, including difficulty translating relevant findings from animal to human models, differences in the timing of neurodevelopment in different species, magnitude and duration of anesthetic exposures, and a lack of physiologic monitoring and surgical stimulation during experiments.1
- SmartTots, a partnership between the US Food and Drug Administration (FDA) and the International Anesthesia Research Society, has proposed standards for ongoing animal research in this field, including a selection of appropriate animal models, anesthetic exposures, and neurobehavioral outcomes and more rigorous study design and data reporting requirements.2
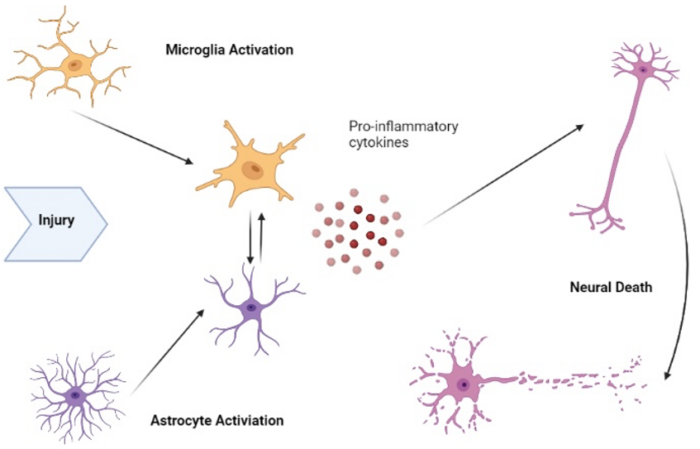
- Key mechanisms by which anesthetics may cause neurotoxicity include apoptosis (programmed cell death), oxidative stress, and neuroinflammation.1 These processes contribute to neuronal damage and cognitive deficits.
Human Data
FDA Response to Evidence3
- In reaction to the preclinical evidence, the FDA released a warning in 2016 about the possible risks of repeated or prolonged exposure (longer than three hours) to general anesthesia in children younger than three years of age and pregnant women.
- However, the FDA acknowledged that anesthetic and sedative drugs are often medically necessary and that untreated pain may also adversely affect neurodevelopment. Therefore, they advised that medical providers should discuss the risks and benefits of anesthetic exposure lasting longer than three hours to pregnant women and children younger than three years and that procedures should potentially be delayed when medically appropriate.
- The FDA has also required labeling changes for specific anesthesia and sedative drugs to include information about the potential risks of neurotoxicity in young children and pregnant women. These changes are intended to provide healthcare providers with important safety information when making treatment decisions.
Human Study Design
- Human studies on anesthesia-induced neurotoxicity in the developing brain have sought to determine whether the adverse effects observed in animal models also apply to children undergoing anesthesia.
- Human studies have primarily been observational studies and retrospective analyses of pre-existing data sets, as conducting controlled, prospective experiments in infants and children is challenging for several reasons, including4
- inability to randomize children to anesthetic and surgical exposures;
- difficulty in accounting for underlying comorbidities; and
- the need to assess outcomes many years after exposure,
- Human studies may be limited by4
- heterogeneity in anesthetic exposures, surgical procedures, and outcome measures;
- use of assessment tools that are not the most sensitive measures for evaluation of neurodevelopment or assessment prior to the development of adverse outcomes;
- lack of pre-exposure and perioperative clinical data, which may allow the inclusion of children with major clinical anomalies or intraoperative complications that can also affect neurodevelopment; and
- limitations of observational and retrospective studies, including unmeasured confounding variables (i.e., socioeconomic status or other environmental variables), incomplete data, and recall bias.
Landmark Studies
- The three most cited studies of anesthesia-induced neurotoxicity in humans are:
- GAS: General anesthesia versus awake regional anesthesia in infancy
- PANDA: Pediatric Anesthesia Neurodevelopmental Assessment
- MASK: Mayo Anesthesia Safety in Kids
- These studies prospectively assessed neurodevelopmental outcomes after exposure to anesthesia in infancy or early childhood. The primary outcome for all three studies was intelligence, but neuropsychological testing of other domains, including executive function and behavior, was also included.5-7
- Outcomes
- These studies showed no difference in intelligence after exposure to anesthesia (primary outcome). However, each study did show some effect on executive and behavioral outcomes.5-7
- When analyzed together, these studies show that a single general anesthetic exposure was associated with statistically significant increases in parent reports of behavioral problems without effects on general intelligence. These scores reflect worse behavioral scores, increased emotional distress, and impaired executive function associated with anesthetic exposure.8
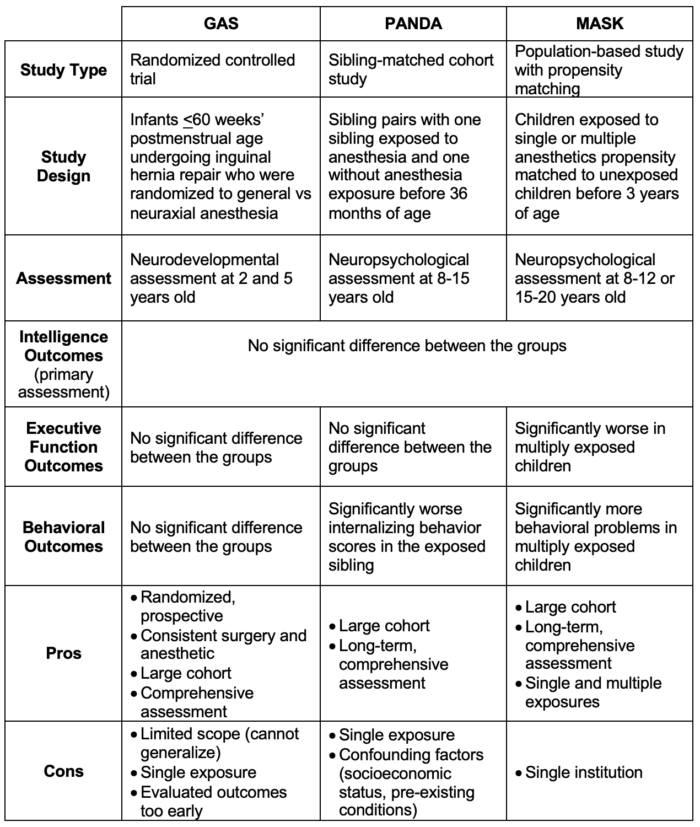
Table 1. Comparison of the GAS, PANDA, and MASK trials5-7
Summary of Human Data
- Many studies have examined anesthesia-induced neurotoxicity in humans. The results have been variable, mainly due to issues already discussed, including the difficulty of creating well-designed trials and the heterogeneity in anesthetic exposure and outcome measures used in these studies.
- A systematic review conducted in 2021 examined 56 published articles to answer four predetermined study questions with the following conclusions:8
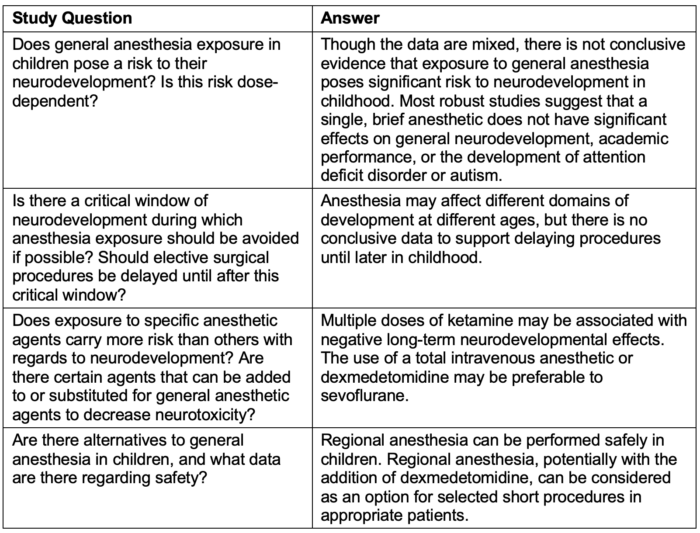
Table 2. Summary of systematic review data from Grabowski et al.8
Future Directions
- Although animal studies have consistently shown adverse neurodevelopmental effects related to anesthesia exposure, human studies have been inconsistent. Further research into the potential neurotoxic effects of anesthetic agents, potential strategies to mitigate these effects, and vulnerable patient populations is warranted.
- Potential avenues for study include:
- Identification of vulnerable patient populations: This may include children who have multiple, lengthy, or prenatal anesthetic exposures; children with congenital anomalies (particularly cardiac anomalies) or significant comorbidities; children of lower socioeconomic status; and children with genetic or environmental factors that predispose them to injury.4
- Identification of intermediate outcomes or biomarkers: No consistent biomarkers of anesthetic-induced neurotoxicity have been identified. However, identifying a biomarker could lead to a better understanding of the mechanisms of neurotoxicity, improved translation from animal to human research, development of targeted protective strategies, and decreased latency from exposure to measurement of outcomes.4
- Investigation of neuroprotective strategies that may mitigate neurotoxic effects of anesthetic agents, including:
- Alternative anesthetic agents: Data from preclinical studies have shown that dexmedetomidine and opioids may have fewer neurotoxic effects than other sedative anesthetic agents. One ongoing study is the TREX (toxicity of remifentanil and dexmedetomidine) trial, which is being conducted by SmartTots. This study will compare a dexmedetomidine-remifentanil-low dose sevoflurane (0.3-0.6% end-tidal) anesthetic compared to standard sevoflurane (2.5-3% end-tidal) anesthetic in children <3 years old undergoing >2 hours of anesthesia.9
- Pharmacologic agents, such as lithium, erythropoietin, and progesterone, may ameliorate neurodegenerative effects.9
- Nonpharmacologic approaches to ameliorate neurotoxic effects.
- Preconditioning: Pre-exposure to subanesthetic doses may induce adaptive responses that protect against higher doses of anesthetics.10
- Environmental enrichment: Postanesthesia environmental enrichment and/or socialization can enhance neuroplasticity and functional recovery.4,10
- Physiologic neuroprotection: This includes many of the strategies we already employ for neuroprotection under anesthesia. They include mild hypothermia, avoidance of hypoxia and hypercarbia, and maintenance of normal blood pressure and glucose levels.9
References
- Bleeser T, Brenders A, Hubble TR, et al. Preclinical evidence for anaesthesia-induced neurotoxicity. Best Practice & Research Clinical Anaesthesiology. 2023; 37(1):16-27. PubMed
- Chinn GA, Pearn ML, Vutskits L, et al. Standards for preclinical research and publications in developmental anesthetic neurotoxicity: expert opinion statement from the SmartTots preclinical working group. British Journal of Anaesthesiology. 2020; 124(5):585-593. PubMed
- FDA Drug and Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. Accessed August 7, 2024. Link
- Ing C, Warner DO, Sun LS, et al. Anesthesia and developing brains: unanswered questions and proposed paths forward. Anesthesiology. 2022; 136(3):500-12. PubMed
- McCann ME, de Graaff JC, Dorris L, et al. GAS Consortium. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019; 393(10172):664-677. PubMed
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016; 315(21):2312-20. PubMed
- Warner DO, Zaccariello MJ, Katusic SK, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: The Mayo anesthesia safety in kids (MASK) study. Anesthesiology. 2018; 129(1):89-105. PubMed
- Grabowski J, Goldin A, Grier Arthur L, et al. The effects of early anesthesia on neurodevelopment: A systematic review. Journal of Pediatric Surgery. 2021; 56(5):851-861. PubMed
- Andropoulos DB. Neuroprotective strategies in anesthesia-induced neurotoxicity. Best Practice & Research Clinical Anaesthesiology. 2023; 37(1):52-62. PubMed
- Lei X, Guo Q, Zhang J. Mechanistic Insights into Neurotoxicity Induced by Anesthetics in the Developing Brain. International Journal of Molecular Sciences. 2012; 13(6):6772-6799. PubMed
Other References
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.