Copy link
Observational Studies
Last updated: 06/21/2024
Key Points
- Observational studies include various designs, including case reports, case series, cross-sectional studies, case-control studies, and cohort studies, each of which is suited for different research purposes.
- Observational studies contribute to medical literature by documenting rare conditions, assessing prevalence, and identifying outcomes and risk factors.
- While these studies provide valuable insights and inform clinical practices, observational studies have limitations, such as their inability to determine causality and potential for bias.
Introduction
- Observational studies are a category of study designs important to medical literature.1 In such studies, the investigator observes and assesses the relationship between exposure and disease outcome to investigate disease progression, outcomes, and risk factors.1
- Observational studies include case reports, case series, cohort studies, case-control studies, and cross-sectional studies (Figure 1).1
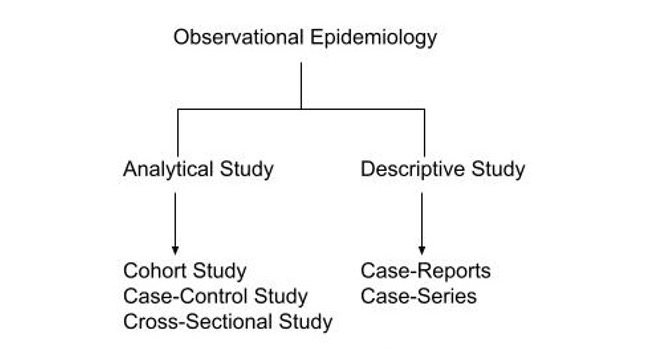
Figure 1. Classification of observational studies
Case Reports
- Case reports are well-established in medical literature and often provide a detailed account of a particular medical case.2 Case reports include information about a patient’s medical history, diagnostic findings, treatments, and outcomes.2 There are several advantages of conducting case reports:2
- They allow clinicians to document new observations.
- They help generate new hypotheses.
- They can serve as an educational tool.
- For example, in the case report by DeLeon et al., the authors highlight the successful use of neuraxial anesthesia in a patient with Fanconi–Bickel syndrome undergoing cesarean delivery.3 This helped to fill a gap in the current literature regarding anesthetic management in such cases. This case report also helps to provide clinicians with insights into managing patients with extremely short stature and rare genetic conditions.3
- There are also limitations associated with case reports:2
- They are unable to provide a cause-and-effect relationship.
- They cannot be generalized to the greater population.
- Therefore, while case reports offer valuable insights, they necessitate cautious interpretation.
Case Series
- Case series also serve an important role in medical research by providing a detailed account of a group of individuals that share a common disease or exposure.4 Data can be collected retrospectively or prospectively without the need for control groups.4 As such, the primary objective of this study design is to characterize the population and their outcomes instead of making comparative assessments.4
Cross-Sectional Studies
- A cross-sectional study is an observational study that analyzes data from a population at a specific point in time.5 These studies are often used to assess the prevalence of health conditions and offer clinicians cost-effective ways to explore associations between variables.5 For example, Aryafar et al. conducted a cross-sectional study demonstrating the usefulness of the brain function index in assessing anesthesia depth in patients undergoing elective laparotomy. This cross-sectional study helps to highlight the potential for improvements in monitoring techniques in anesthesia management.6
- However, cross-sectional studies have several limitations.5
- They are unable to determine causality since temporality is unknown.
- Rare conditions cannot be effectively studied.
- These limitations highlight the importance of careful consideration of study design in research, emphasizing the need for further comparative designs to validate findings for comprehensive assessment within anesthesiology research.
Case-Control Studies
- Case-control studies are another type of observational study commonly used to examine factors associated with outcomes or conditions.5 In a case-control study, the investigators categorize subjects as cases (those with the outcome of interest) or controls (those without the outcome of interest but are similar to cases).5 Data related to exposure to risk factors are then collected retrospectively through data abstraction of records or interviews (Figure 2).5
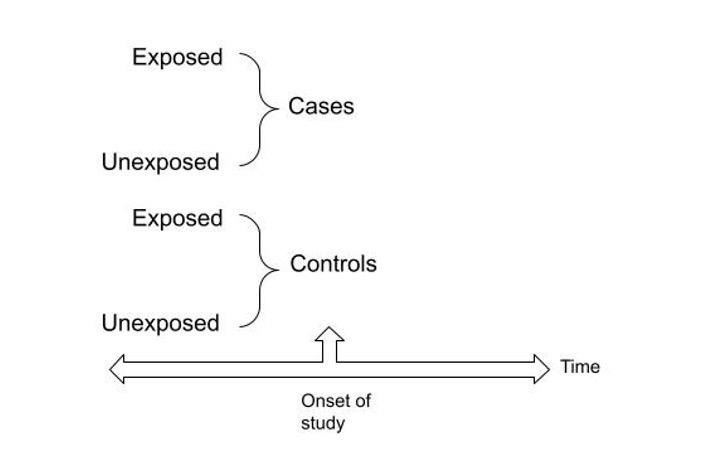
Figure 2. Diagram presenting the design of case-control study
- Case-control studies have several advantages.5
- They are relatively inexpensive and easy to conduct.
- They are suitable for examining rare outcomes.
- They allow investigators to examine multiple risk factors.
- For example, Wu et al. show the utility of case-control studies in anesthesiology.7 By comparing patients that experienced arytenoid dislocation with their matched controls, the researchers highlighted the protective effect of intubation stylet use and the increased risk associated with prolonged operation time.7 Such studies play a critical role in informing clinical practices and enhancing patient safety in anesthesia management.7
- However, there are limitations to case-control studies.8
- Selecting appropriate comparison groups can be difficult.
- Validating information can be challenging.
- They are prone to recall bias, where participants may not accurately remember events.
- They are prone to information bias, where data may be inaccurate/inconsistent.
- Despite these limitations, case-control studies remain valuable tools in epidemiological research.
Cohort Studies
- Cohort studies are another type of observation study that is commonly used to determine the incidence and natural history of a disease.5 In cohort studies, participants are selected based on exposure status and followed up over a period until the outcome of interest occurs (Figure 3).5
- Advantages of cohort studies include5,8
- Ability to assess causality due to the temporal framework
- Examination of rare exposures
- Ability to calculate disease rates among exposed and unexposed groups over time
- Limitations of cohort studies include8
- Requirement for a large population of subjects
- Requirement for long follow-up periods
- Loss of subjects to follow-up
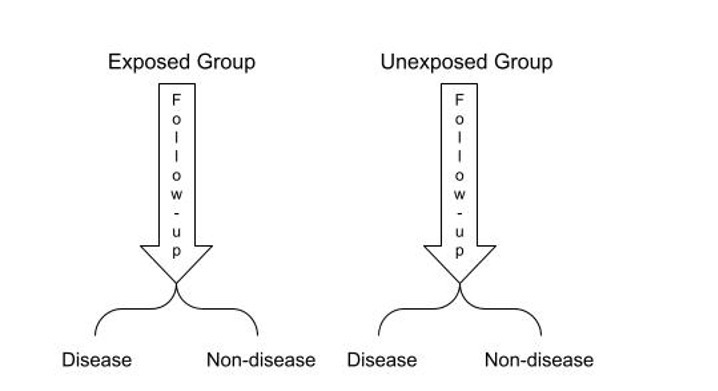
Figure 3. Diagram presenting the design of a prospective cohort study
- Cohort studies may be prospective or retrospective.5 In a prospective cohort study, participants without the outcome of interest are selected and followed up over a period to observe if they develop the outcome of interest (Figure 3).5 In a retrospective cohort study, the study design is the same; however, the data is all collected from past records.5
- For example, DiMaggio et al. highlight the significance of cohort studies. In this retrospective cohort study, researchers investigated the long-term effects of surgical and anesthetic exposures on neurodevelopment.9 By following a cohort of children since birth, DiMaggio et al. identified a significant association between hernia repair before three years of age and an increased risk of behavioral and developmental disorders.9 These findings emphasize the importance of cohort studies to help inform clinical practice regarding anesthesia safety in pediatric populations.9
- Observational studies play a critical role in medical research and offer valuable insights into the natural history, prevalence, and risk factors of various diseases.1 Through various observational designs, researchers can gather evidence that helps to inform practices and policy-making.1 Each design has its strengths and weaknesses (Table 1), making it essential for investigators to choose the design based on their research questions.1 Despite this, observational studies remain essential to advancing knowledge and improving health outcomes.1
- Several initiatives have been undertaken to improve the conduct and reporting of these observational studies. One example is the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.10 Link
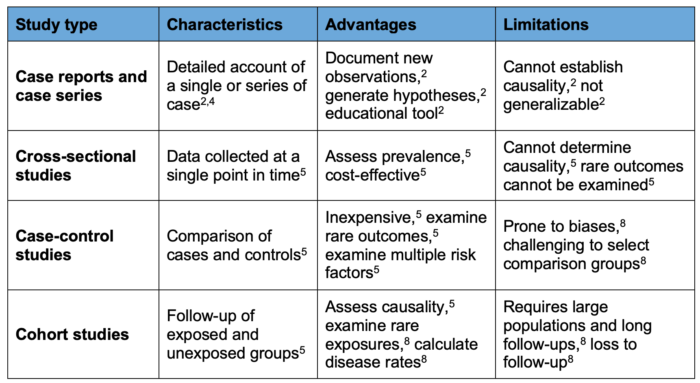
Table 1. Comparison summary of observational study designs
References
- Gilmartin-Thomas JF, Liew D, Hopper I. Observational studies and their utility for practice. Aust Prescr. 2018;41(3):82-85. PubMed
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014; 7:264. PubMed
- DeLeon AM, Gaiha RD, Peralta FM. The successful anesthetic management of a cesarean delivery in a patient with Fanconi-Bickel syndrome. Case Rep Anesthesiol. 2022;3220486. PubMed
- Torres-Duque CA, Patino CM, Ferreira JC. Case series: an essential study design to build knowledge and pose hypotheses for rare and new diseases. J Bras Pneumol. 2020;46(4): e20200389. PubMed
- Mann CJ. Observational research methods. Research design II: cohort, cross-sectional, and case-control studies. Emerg Med J. 2003;20(1):54-60. PubMed
- Aryafar M, Bozorgmehr R, Alizadeh R, Gholami F. A cross-sectional study on monitoring depth of anesthesia using brain function index among elective laparotomy patients. International Journal of Surgery Open. 2020; 27:98-102. Link
- Wu L, Shen L, Zhang Y, Zhang X, Huang Y. Association between the use of a stylet in endotracheal intubation and postoperative arytenoid dislocation: a case-control study. BMC Anesthesiol. 2018;18(1):59. PubMed
- Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234-42. PubMed
- DiMaggio C, Sun LS, Kakavouli A, et al. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21(4):286-91. PubMed
- Von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4(10): e296. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.