Copy link
Multimodal Analgesia
Last updated: 09/11/2024
Key Points
- The goal of multimodal analgesia is to improve pain control and minimize adverse side effects.
- Specifically, this approach is designed to reduce opioid consumption, thereby mitigating the risk of respiratory depression, tolerance, and opioid use disorder, as well as decreasing overall opioid exposure postoperatively in the community.
- Multimodal analgesia targets different components of the pain pathway: transmission, transduction, modulation, and perception. This approach treats peripheral nociception and reduces central sensitization.1
Introduction
- The current paradigm of perioperative pain management is called multimodal analgesia. Treatment for surgical pain begins preoperatively, is managed intraoperatively by the anesthesiologist, and continues postoperatively. Multimodal analgesia aims to target multiple receptors that modulate pain and minimize unwanted side effects.
- Postsurgical pain that is not adequately controlled can lead to worse patient outcomes, including increased morbidity, delayed healing, decreased function, development of chronic pain, and poor patient experience.2
- Multimodal analgesia involves using various medications targeting different ascending and descending pain pathways (Figure 1).

Figure 1. Medications for multimodal analgesia
- Several neurotransmitters are known to be involved in the transmission of pain. By targeting different receptors, pain can be reduced through various mechanisms. Pain pathways involve discrete anatomical and physiological processes, and, by targeting different parts of the pain pathways, multimodal pain regimens can offer better pain control (Figure 2).
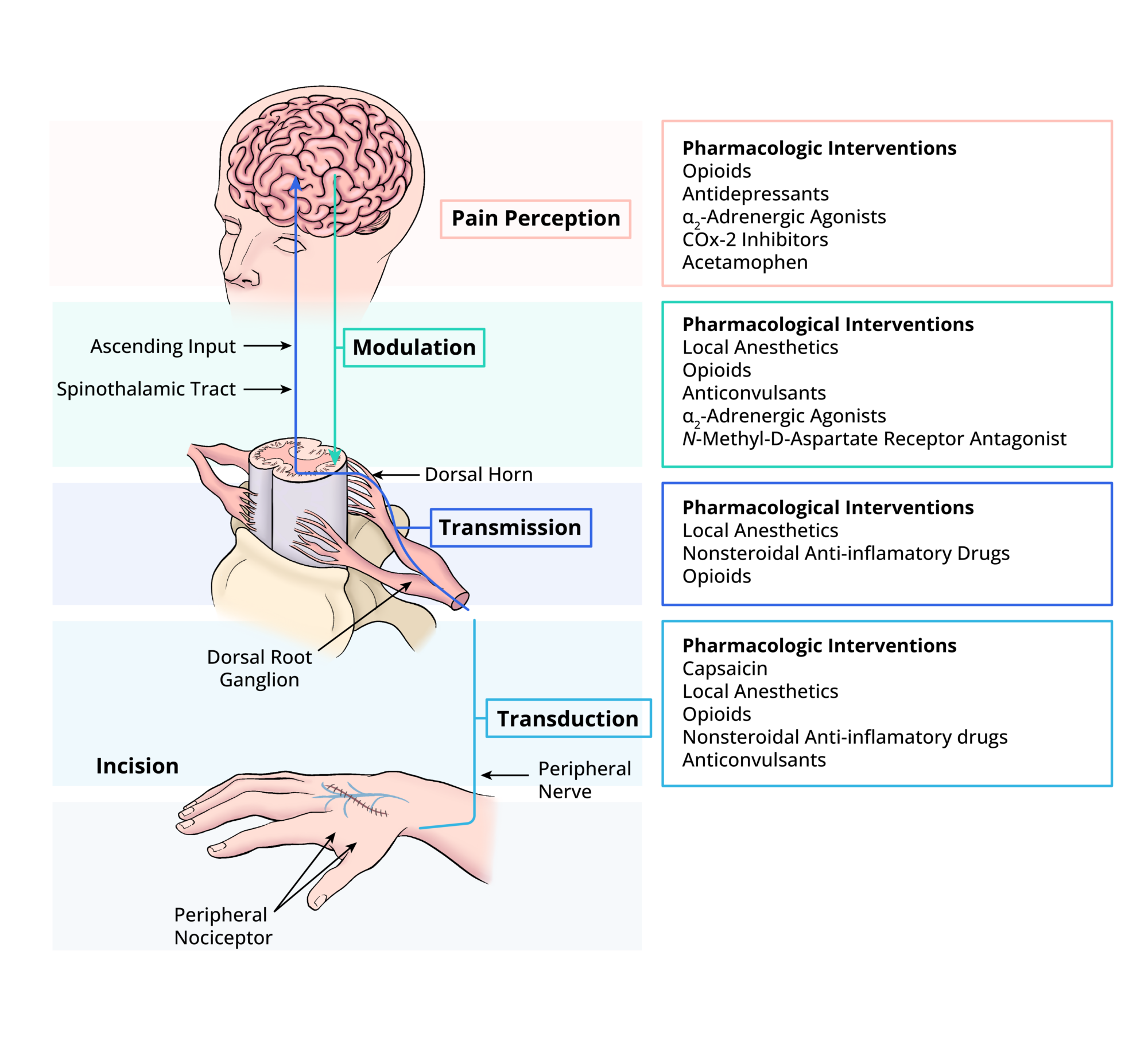
Figure 2. Acute pain is processed initially from the periphery to the central nervous system. Along each part of the pathway, specific medications and techniques can ultimately reduce the amount of pain experienced by the patient.
Medications
- A thorough preoperative assessment of patients is critical to developing a successful multimodal analgesic plan. By determining which medications are appropriate in the perioperative period, an anesthesiologist can improve pain control and reduce the risk of unwanted side effects.
- Each class of medication has risks and benefits. Patient comorbidities and surgical approaches should be considered when deciding on the appropriate pain regimen. When used with a thoughtful multimodal approach, they can help patients have a better perioperative experience.
Opioids
- Historically, opioids are the core of perioperative pain management. When used with adjunctive therapies, they play a crucial role in treating acute pain.
- Opioids bind to specific G protein-coupled receptors. Presynaptically, they block calcium channels to inhibit the release of substance P and glutamate, therefore altering nociception. Postsynaptically, they open potassium channels, hyperpolarizing cell membranes.3 This increases the action potential required to transmit pain.
- Opioids can activate both peripheral and central opioid receptors. Four classes of opioid receptors (mu, kappa, nociception, and delta) are involved in mediating analgesia.
- Buprenorphine is a partial mu-opioid receptor agonist and an antagonist at the kappa-opioid receptor. Tramadol has mixed mechanisms of action that include blocking monoamine uptake.
- Tapentadol is an opioid receptor agonist and a norepinephrine reuptake inhibitor.
- Current practice includes the use of agonists, partial agonists, and agonist-antagonists for pain control.
- Adverse side effects include respiratory depression, nausea, vomiting, pruritis, urinary retention, constipation, euphoria, and risk of developing opioid use disorder.
- Please see the OA summary “Opioids: Basics” for more details. Link
Local Anesthetics
- Local anesthetics can be administered topically, intravenously, subcutaneously, epidurally, intrathecally, or deposited around specific nerves. They are commonly used perioperatively through peripheral nerve blocks and neuraxial injections.
- Local anesthetics bind ion channels on cell membranes and prevent the influx of sodium into a cell. They preferentially bind to channels in the open or inactive state versus the resting state. When sodium channels are in the resting state, sodium cannot traverse the membrane. In the active state, the channel is open, allowing sodium to enter the cell and cause depolarization. This causes a change in membrane voltage, which closes the channel and leads to an inactive state. Sodium is transported out of the cell, resulting in cell hyperpolarization.4
- Local anesthetic properties depend on several factors. Potency and onset are related to lipid solubility, allowing for quicker and enhanced diffusion. Duration of action is related to protein affinity. Local anesthetic toxicity is dose-dependent and can result in central nervous system (CNS) depression, resulting in death.
N-methyl-D-aspartate (NMDA) Antagonists
- Ketamine is an NMDA antagonist. It partially inhibits glutamate receptors and activates mu-opioid receptors, contributing further to its analgesic properties. It is beneficial for patients who have a history of opioid tolerance or who are undergoing procedures that typically result in severe pain postoperatively. It has also been shown to decrease opioid consumption in the acute postoperative period and is thought to reduce the risk of developing central sensitization.5
- It provides analgesia at lower doses and can be used as an anesthetic at high doses.
- Side effects include dysphoria, hallucinations, nausea, tachycardia, and hypertension. Caution when used in patients with preexisting coronary artery disease and uncontrolled hypertension due to its blockade of catecholamine reuptake.
- Ketamine is derived from cyclohexanone. CYP3A4 is responsible for most of ketamine’s metabolism. Intravenous ketamine has a rapid onset of action as it enters the CNS quickly. From there, it redistributes to peripheral tissue and is eliminated by the kidneys.6
- Please see the OA summary on ketamine for acute and chronic pain management for more details. Link
Alpha-2 Agonists
- These medications affect the sympathetic nervous system by selectively binding adrenergic receptors to produce sympatholytic effects. There are two types of adrenergic receptors: alpha and beta. Alpha receptors can be further divided into alpha-1 and alpha-2 receptors, each with three subtypes. Alpha 2 receptors are G-coupled proteins, and most of them are located in the CNS. Agonism of the receptor results in analgesia and sedation.7
- Dexmedetomidine binds to receptors found on neurons in the locus ceruleus inhibiting them which prevents the release of norepinephrine. It also results in the activation of descending pathways due to the release of acetylcholine at the dorsal horn.
- Clonidine and dexmedetomidine can be used as adjuncts to local anesthetics. Clonidine blocks A-delta and C fibers, decreasing the time of the block’s onset and increasing the density of sensory blockade.
- Tizanidine is also an alpha-2 agonist and is used as a muscle relaxant.
- Dose-dependent hypotension, bradycardia, and sedation are all potential side effects from these medications and, therefore, should be used with caution in elderly patients.
- Please see the OA summary on dexmedetomidine for more details. Link
Calcium Channel Blockers
- Calcium channel blockers stabilize membrane ion channels and can amplify GABA to reduce pain transmission or blunt glutamate amplification, an excitatory neurotransmitter.
- Gabapentin is the most common membrane-stabilizing medication perioperatively. It is an analog of GABA that blocks the α2δ subunit of voltage-gated calcium channels in the dorsal root ganglion. Side effects include somnolence, dizziness, and gait disturbance.8
Muscle Relaxants
- Antispasmodics act on the CNS rather than directly on skeletal muscle. Therefore, calling them muscle relaxants is a misnomer, as they do not directly affect peripheral muscles.
- Cyclobenzaprine and methocarbamol can be used as part of a multimodal approach to pain management. The mechanism of action of methocarbamol is not completely understood; it is believed to act on the CNS. Cyclobenzaprine is a centrally acting muscle relaxant and a 5-HT2 receptor antagonist, giving it its antispasmodic properties. It can be used to treat muscle spasms but will not improve spasticity related to cerebral or spinal cord pathologies. Structurally, it is related to tricyclic antidepressants.
- Magnesium centrally acts as an NMDA antagonist and mediates calcium influx into skeletal muscle cells. Similarly to ketamine, another NMDA antagonist, magnesium is thought to decrease central sensitization.9
- As with many other pain medications, medications in this category can cause sedation.
NSAIDs
- Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit the prostaglandin pathway. They do this by blocking cyclooxygenase, a key enzyme in converting arachidonic acid into mediators such as prostaglandin. This, in turn, decreases inflammation-mediated pain in response to tissue injury. NSAIDs are categorized as nonselective or COX-2 specific.10
- NSAIDs increase the risk of bleeding due to antiplatelet activity. Prostaglandins protect the gastric mucosa; thus, there is an increased risk of ulcers with the potential blocking of COX-1. In patients with any degree of renal dysfunction, decreased prostaglandin production can harm kidney hemodynamics and worsen function. Bone healing can also be delayed by prolonged use of NSAIDs.
- Ketorolac inhibits COX-1 more than COX-2, making it more likely to cause gastrointestinal (GI) complications. Celecoxib and meloxicam are COX-2 selective and less likely to have GI effects.
- The use of NSAIDs must be discussed with the surgical team before administration.
- Please see the OA summary on NSAIDs for more details. Link
Acetaminophen
- The actual mechanism of action of acetaminophen is unknown. It is thought to inhibit centrally mediated COX pathways, resulting in analgesia. It is also believed to inhibit NMDA receptors, decrease nitric oxide production, and increase endogenous cannabinoids.
- Perioperatively, this can be given orally or intravenously. Intravenous acetaminophen can be cost-prohibitive. Acetaminophen has few side effects when it is dosed appropriately. However, high doses can lead to hepatic failure secondary to severe liver necrosis.
- Acetaminophen can be given to patients with chronic liver disease, albeit at a reduced dose of 2-3 g per day in adults.
- Please see the OA summary on acetaminophen for more details. Link
References
- Schug S. A multimodal approach to managing postoperative pain. Pain Management Today. 2019; 6(2): 55-8. Link
- Pirie K, Traer E, Finniss D, Myles P, Riedel B. Current approaches to acute postoperative pain management after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 2022; 129(3): 378-93. PubMed
- Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012;6(1):11-6. PubMed
- Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesthesia Progress. 2012;59(2):90-101. PubMed
- Kissin I, Bright C, Bradley E. The effect of ketamine on opioid-induced acute tolerance: can it explain the reduction of opioid consumption with ketamine-opioid analgesic combinations? Anesth Analg. 2000; 91(6): 1483-8. PubMed
- Rosenbaum SB, Gupta V, Patel P. Ketamine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Link
- Giovannitti JA Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog 2015;62(1):31-9. PubMed
- Chincholkar M. Gabapentinoids: pharmacokinetics, pharmacodynamics, and considerations for clinical practice. Br J Pain. 2020;14(2):104-14. PubMed
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation, implications for the treatment of post-injury pain hypersensitivity states. Pain.1991;44:293–9. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.