Copy link
Immune Thrombocytopenia
Last updated: 04/04/2023
Key Points
- Immune thrombocytopenia (ITP) is a bleeding disorder characterized by a platelet count of less than 100,000 plt/μl in the absence of other primary causes of thrombocytopenia.
- The pathogenesis of ITP involves an immunologic loss of tolerance to platelets with autoantibodies to glycoproteins IIb/IIIa, resulting in splenic destruction of platelets and clinical thrombocytopenia.
- The clinical manifestations of ITP are related to bleeding and can range from petechia and purpura to severe hemorrhage and death, especially with platelet counts less than 10-20,000 plt/μl.
- The primary treatment options for intraoperative ITP-related bleeding include intravenous immunoglobulin (IVIg) and corticosteroids; however, other options are available, including newer thrombopoietin-receptor agonists (TPO-RA), immunomodulators, and possibly splenectomy.
Introduction
- Immune thrombocytopenia (ITP) is a bleeding disorder characterized by isolated thrombocytopenia, generally defined as a platelet count less than 100,000 plt/μl in the absence of other causes of thrombocytopenia.1,2
- ITP has previously been called idiopathic thrombocytopenic purpura or immune thrombocytopenic purpura; however, these terms have largely been replaced by “immune thrombocytopenia” in accordance with a consensus statement to reflect the known autoantibody mechanism and the absence of purpura in most patients.3
- The incidence of primary ITP in adults is 3.3 in 100,000 adults per year, with a predilection for younger female patients, though the prevalence in older age is roughly even between men and women.3
- The disease process in children tends to be more short-lived with spontaneous recovery (generally less than 6 months), whereas ITP in adults typically has a more insidious onset and follows a chronic course.
- ITP may be primary or secondary. Primary ITP is characterized by isolated thrombocytopenia, and secondary ITP is due to secondary causes, such as autoimmune disorder, viral infection, etc.4
- Signs and symptoms may vary, but the predominant clinical manifestations are mostly related to bleeding and can range from minor bleeding or bruising to severe hemorrhage.
Pathophysiology
- The pathophysiology of primary ITP is thought to be mediated by the loss of tolerance to glycoproteins expressed on the platelet or megakaryocyte.4 In 1951, Harrington and Hollingsworth discovered that thrombocytopenia could be induced in healthy volunteers by injecting plasma from ITP-affected individuals, suggesting a humoral component. The most commonly implicated targets of these antibodies are platelet glycoproteins (GP) IIb/IIIa and Ib/IX.5
- While there are myriad immunologic factors involved in the pathogenesis of ITP, the predominant dysregulation is thought to be a T-cell-dependent antigen-driven clonal expansion and somatic mutation, resulting in IgG autoantibodies to platelet proteins.1 The exact inciting mechanism responsible for this loss of tolerance remains largely unknown but likely involves a defect in antigen-presenting cells (APCs) which begins the downstream stimulation of antibody production and cytotoxic T-cell activation.2 Once activated T and B cells product autoantibodies against platelet glycoproteins GPIIb/IIIa and Ib/IX. These antibody-coated platelets are then destroyed by phagocytes, primarily in the spleen, resulting in clinical thrombocytopenia.
- Patients with ITP also have abnormalities of immune mediators such as cytokines and serum complement, suggesting a complex interplay between soluble factors and regulatory cells. This multifactorial process may explain why up to 50% of ITP patients do not have detectable autoantibodies and that remission of ITP can occur even in the presence of continued antibodies.5
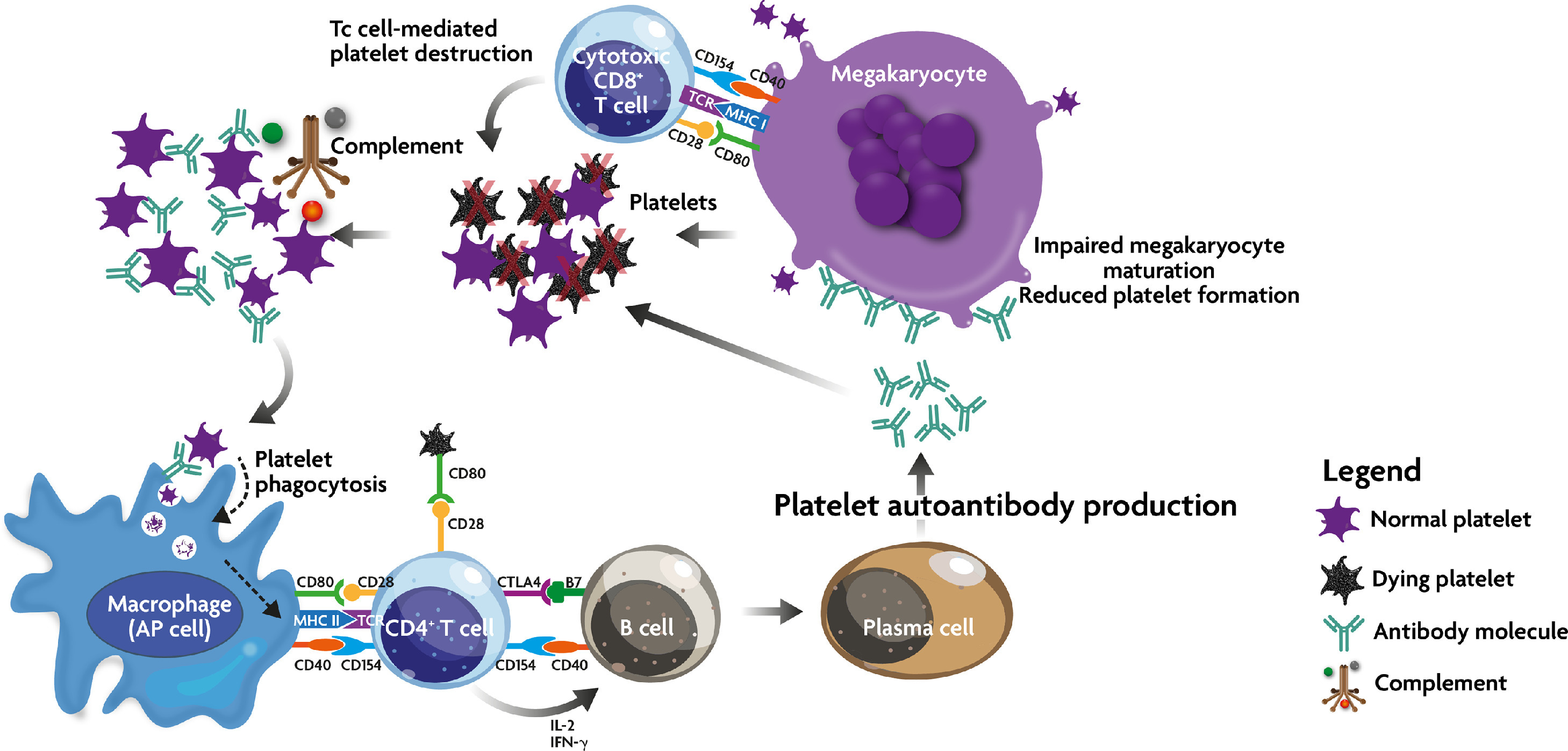
Figure 1. Immune effector mechanism in ITP. Due to a breakdown in self-tolerance, APC (including megakaryocytes) process and present platelet autoantigens to autoreactive T cells, which then begin a cascade of events, including stimulation of autoantibody production and cytotoxic T cell activation. These two mechanisms lead to peripheral platelet destruction and megakaryocyte inhibition in the bone marrow. In addition, autoantibody-opsonized platelets may come under the attack of the complement cascade. Source: Provan D, Semple JW. Recent advances in the mechanisms and treatment of immune thrombocytopenia. EBioMedicine, 2022;76:103820. CC BY-NC-ND 4.0.
- The pathogenesis of secondary ITP is similar to primary ITP, except the inciting factor for APC dysregulation can be attributed to a secondary causes, such as autoimmune disorders or infection. Common causes of secondary ITP are listed below:
Autoimmune Conditions
- Systemic lupus erythematosus (SLE)
- Antiphospholipid syndrome (APS)
- Rheumatoid arthritis
Infections
- Human immunodeficiency virus (HIV)
- Helicobacter pylori
- Varicella zoster virus (VZV)
- Cytomegalovirus (CMV)
- Hepatitis C virus (HCV)
Immunological Dysfunction
- Lymphoma, leukemia
- Common variable immune deficiency (CVID)
Diagnosis
- The diagnosis of ITP requires that other causes of thrombocytopenia be excluded. A thorough history and physical exam can help elucidate common causes of thrombocytopenia (i.e., drug-mediated, kidney, or liver dysfunction). While a complete blood count and peripheral blood smear are the only requisite lab work to diagnose ITP, other studies are often ordered to help rule out secondary causes of thrombocytopenia (see Tables 1 and 2 below).
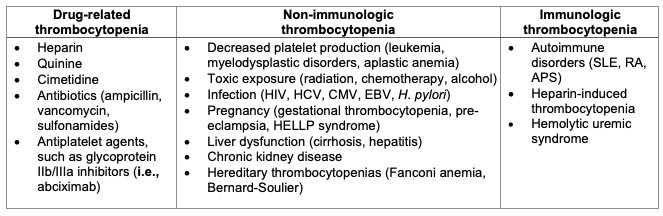
Table 1. Differential diagnosis of ITP. Adapted from Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495-520.1
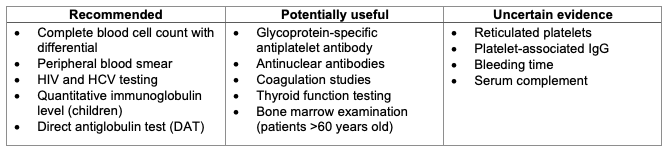
Table 2. Laboratory testing in patients with ITP. Adapted from Provan D, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817.8
Clinical Presentation
- The most common clinical features of ITP are secondary to low platelet count and include bleeding, fatigue, and rarely, thrombosis. The severity of bleeding is variable and often correlates with the absolute platelet count, where below 20,000 plt/μl increases the risk of epistaxis, petechiae, or ecchymoses.
- Spontaneous mucosal bleeding often occurs under 10,000 plt/μl, and fewer than 5,000 plt/μl can result in catastrophic bleeding such as intracranial hemorrhage (ICH) and severe internal bleeding.4 The frequency of ICH tends to be higher in adults than in children (1.4% vs. 0.4% in one review), but the rates of severe, non-ICH bleeding is higher overall in children (20.2% vs. 9.6%).6
- Predictors of severe bleeding include platelet count below 10-20,000 plt/μl, chronic ITP (longer than 12 months), concurrent nonsteroidal anti-inflammatory drugs use, and female sex.
- Fatigue is a common symptom of ITP and may be related to thrombocytopenia itself or comorbid conditions, as some individuals endorse fatigue even when the platelet count is only mildly reduced.
- Lastly, thrombocytopenia does not necessarily preclude thrombosis, which can rarely occur through unknown mechanisms. Some studies postulate a slightly increased risk of thrombosis, but it is unclear as to whether this is due to the pathogenesis of ITP itself or secondary to associated conditions (SLE, APS) and treatments (steroids, splenectomy).7
Perioperative Management
Preoperative Evaluation
- The goal of anesthetic management in the preoperative setting focuses on optimizing the platelet count. The most common treatment options are outlined below in Table 3. It is important to note that these treatments should ideally be started in the outpatient setting, as most take days to weeks for full therapeutic effect. For most patients, the treatment goal is to maintain a platelet count greater than 20-30,000 plt/μl to prevent the risk of major bleeding; however, this target should be tailored to individual patient needs, especially in the perioperative setting.
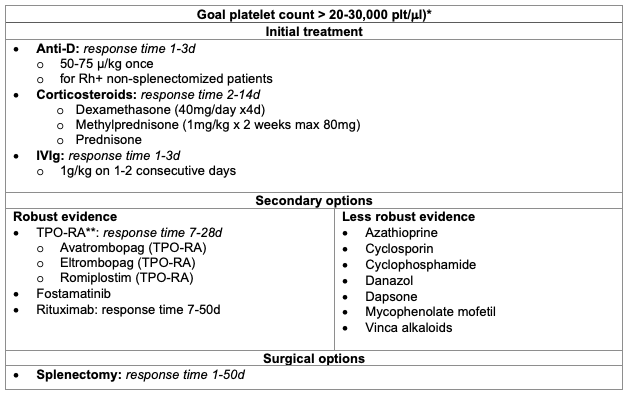
Table 3. Treatment options for ITP.
*For patients not undergoing surgeries or procedures
**TPO-RA: thrombopoietin receptor agonist
Adapted from Provan D, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817.8
- The goal platelet level for a surgery or procedure depends on the degree of invasiveness, length of the procedure, and patient comorbidities. General recommendations for surgery or medical therapy in adults are outlined below:8,9
- Dental prophylaxis and simple extractions: ≥ 30,000 plt/μl
- Complex dental extractions: ≥ 50,000 plt/μl
- Minor surgery (e.g., cataract with laser): ≥ 50,000 plt/μl
- Obstetric vaginal delivery (uncomplicated): ≥ 50,000 plt/μl
- Neuraxial anesthesia: ≥ 70,000 plt/μl
- between 50-70,000 plt/μl there is limited data, suggest risk vs. benefit comparison
- Major nonneuraxial surgery: ≥ 80,000 plt/μl
- Neurosurgery: ≥ 100,000 plt/μl
Laboratory Investigations
- A platelet count and type and screen are the most important labs to obtain prior to surgery. Additional tests may be considered to provide a more robust clinical picture. Some studies suggest that thromboelastogram (TEG) or rotational thromboelastometry (ROTEM) may be better predictors of clinical bleeding than the total platelet count.10 Additionally, coagulation studies such as prothrombin time, partial thromboplastin time, international normalized ratio, and fibrinogen may be useful if there are comorbidities contributing to coagulopathy, such as liver dysfunction.
Intraoperative Considerations
- General versus regional anesthesia
- The decision to proceed with regional anesthesia versus general anesthesia must primarily consider the risk of bleeding, both with the surgery and the anesthetic technique.
- As outlined above, recommendations for neuraxial anesthesia suggest a platelet count greater than 70,000 plt/μl. Although the American Society of Regional Anesthesia (ASRA) guidelines do not provide specific platelet thresholds for nonneuraxial anesthesia, a platelet count greater than 50,000 plt/μl is generally considered safe for extremity and truncal blocks excluding neuraxial, deep plexus, or incompressible sites.11
- There is insufficient evidence as to the superiority of endotracheal intubation versus supraglottic airway devices regarding the risk of airway/oropharyngeal bleeding; however, nasal intubation should generally be avoided, if possible.
- Lines/Monitors
- Lines and monitors should be tailored to the individual patient and procedure, but at a minimum, 1-2 large bore IVs are recommended in the event resuscitation is required.
- An arterial line might prove useful for frequent lab draws and close hemodynamic monitoring.
- More advanced lines and monitors, such as a central line or transesophageal echocardiography, should be considered on a case-by-case basis.
- Emergency bleeding
- Standard resuscitation with blood products as needed PLUS
- IVIg (1 g/kg x 1-2 doses in adults, 0.8-1 g/kg in children) PLUS
- Corticosteroids (IV methylprednisolone 30mg/kg/day)
Consider: - TPO-RA (e.g., eltrombopag)
- Possible splenectomy if refractory bleeding
- Antifibrinolytics are possibly useful (grade C recommendation)
- Tranexamic acid 1g every 8 hours
- Epsilon-aminocaproic acid 1-4g every 6 hours
- Standard resuscitation with blood products as needed PLUS
Postoperative Care
Postoperative care is dependent on the surgical procedure and intraoperative course but should focus on continuous monitoring of postoperative bleeding and close hemodynamic monitoring.
References
- Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495-520. PubMed
- Provan D, Semple JW. Recent advances in the mechanisms and treatment of immune thrombocytopenia. EBioMedicine. 2022;76:103820. PubMed
- Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829-35. PubMed
- Onisai M, Vladareanu AM, Spinu A, et al. Idiopathic thrombocytopenic purpura (ITP) - new era for an old disease. Rom J Intern Med. 2019;57(4):273-83. PubMed
- Stasi R. Pathophysiology and therapeutic options in primary immune thrombocytopenia. Blood Transfus. 2011;9(3):262-73. PubMed
- Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13(3):457-64. PubMed
- Ali EA, Rasheed M, Al-Sadi A, et al. Immune thrombocytopenic purpura and paradoxical thrombosis: A systematic review of case reports. Cureus. 2022;14(10):e30279. PubMed
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817. PubMed
- Bauer ME, Arendt K, Beilin Y, et al. The Society for Obstetric Anesthesia and Perinatology interdisciplinary consensus statement on neuraxial procedures in obstetric patients with thrombocytopenia. Anesth Analg. 2021;132(6):1531-44. PubMed
- Nguyen TH, Tran TT, Hoang TT, et al. Rotational thromboelastometry parameters as predicting factors for bleeding in immune thrombocytopenic purpura. Hematol Oncol Stem Cell Ther. 2021;14(1):27-32. PubMed
- Ashken T, West S. Regional anaesthesia in patients at risk of bleeding. BJA Education. 2021;21(3):84-94. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.