Copy link
Heparin-Induced Thrombocytopenia and Thrombosis
Last updated: 07/11/2024
Key Points
- Heparin-induced thrombocytopenia (HIT) is a serious complication of heparin therapy characterized by a decrease in platelet count (thrombocytopenia) and an increased risk of complications from blood clot formation (thrombosis).
- HIT typically occurs 5 to 10 days after exposure to any form or amount of heparin products, although it can manifest earlier in patients who have previously been sensitized to heparin.
- Clinical suspicion is paramount in diagnosing HIT, particularly in patients on heparin therapy who develop a sudden, unexplained drop in platelet count or new thrombotic events.
Epidemiology
- Up to 8% of patients receiving heparin are at risk of developing HIT antibodies, but this does not necessarily mean they will develop the clinical syndrome of HIT. HIT is estimated to occur in approximately 1-5% of patients exposed to heparin. However, rates may vary based on factors such as the type of heparin (unfractionated vs. low molecular weight), duration of exposure, and patient-specific risk factors.1
- The incidence is higher with unfractionated heparin (UFH) than low molecular weight heparin (LMWH). However, antibodies developing in patients receiving UFH frequently cross-react with LMWH. Additionally, specific patient populations are at higher risk, including those undergoing cardiac surgery, orthopedic surgery, or receiving heparin for thromboprophylaxis.2
- Despite its relatively low incidence, HIT is associated with significant morbidity and mortality due to the potential for life-threatening thrombotic complications if not promptly recognized and managed.
- Additionally, heparin is very commonly used. HIT should be considered even in patients with a prior history of heparin exposure, as sensitization can occur with each subsequent exposure. Prompt recognition and appropriate management are essential to prevent thromboembolic events and minimize patient morbidity and mortality.
Diagnosis
Diagnosing HIT requires clinical assessment, laboratory testing, and exclusion of alternative causes of thrombocytopenia.
Clinical Assessment
- The hallmark of HIT is a decrease in platelet count, typically occurring 5 to 10 days after heparin exposure, though it can manifest earlier in sensitized individuals.
- Thrombotic complications may also be present, such as deep vein thrombosis, pulmonary embolism, or arterial thrombosis.
- Clinical suspicion should be high in patients with unexplained thrombocytopenia or new thrombotic events while on heparin therapy.3
Laboratory Testing
- Laboratory confirmation of HIT is essential for diagnosis and typically involves HIT antibody assays.
- The gold-standard assays include the serotonin release assay (SRA) and the heparin-induced platelet activation (HIPA) assay. These tests detect antibodies that bind to platelet factor 4 (PF4)/heparin complexes, triggering platelet activation and the release of serotonin.
- Enzyme-linked immunosorbent assays (ELISA) are also available and are more widely used due to their convenience, although they may have lower sensitivity and specificity than functional assays.
Exclusion of Alternative Causes
- Thrombocytopenia can have various etiologies, including sepsis, medications, disseminated intravascular coagulation (DIC), and other immune-mediated disorders.4
- Clinicians should perform a thorough evaluation to exclude alternative causes of thrombocytopenia before diagnosing HIT.
Pathophysiology of HIT
- PF4 is a small molecule stored in alpha-granules of platelets and released upon activation (Figure 1) PF4 is positively charged and can bind to negatively charged heparan (a heparin-like substance normally present on the endothelial cell surface) or exogenous heparin with a much higher affinity than heparan.
- PF4 + heparin → can trigger the formation of IgG, IgA, or IgM antibodies specific to the heparin-PF4 complex. If IgG, while attached to the heparin-PF4 complex, binds to the FC receptor on the platelet surface, this can lead to platelet activation and release of pro-thrombotic substances (e.g., thrombin) and PF4.
- If more PF4 is released, more heparin-PF4 complexes can form, activating more platelets.
- Macrophage consumption of the IgG-coated platelets and removal by reticuloendothelial system → thrombocytopenia
- Platelet activation → platelet aggregation → thrombocytopenia + thrombosis
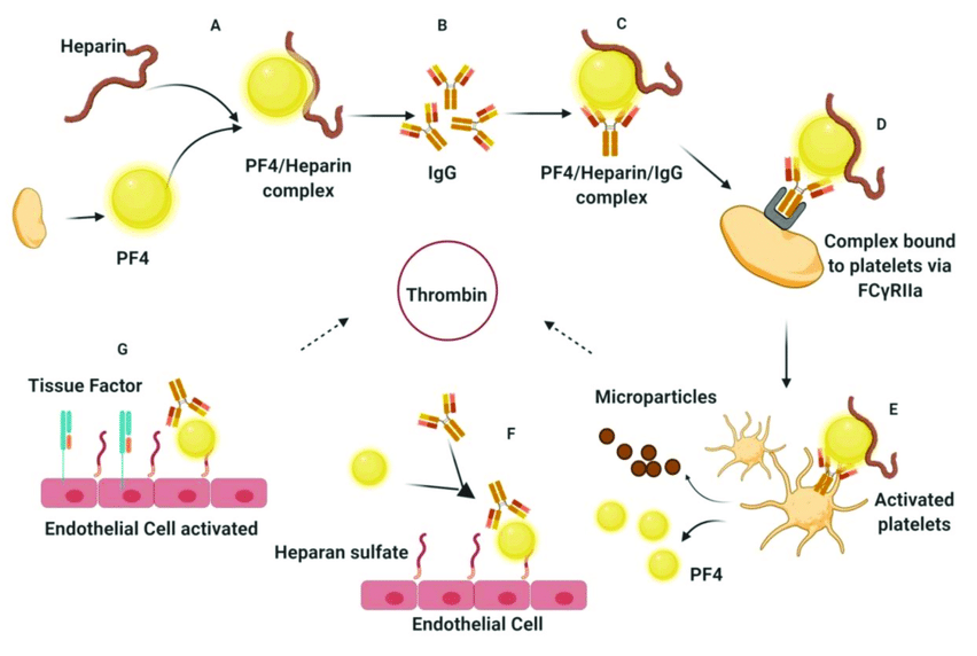
Figure 1. Pathophysiology of HIT. Source: Dwivedi R, et al. Marine antithrombotics. Mar Drugs. 2020;18(10): 514. CC BY 4.0.
4Ts Score
- The 4Ts score is the most widely utilized HIT pretest probability scoring system. It takes into consideration the degree and timing of thrombocytopenia, the development of thrombosis, and the potential for other causes of thrombocytopenia (Table 1).
- A meta-analysis demonstrated that low probability scores (£ 3) have good negative predictive value, but the positive predictive value for high and intermediate scores is less useful.6
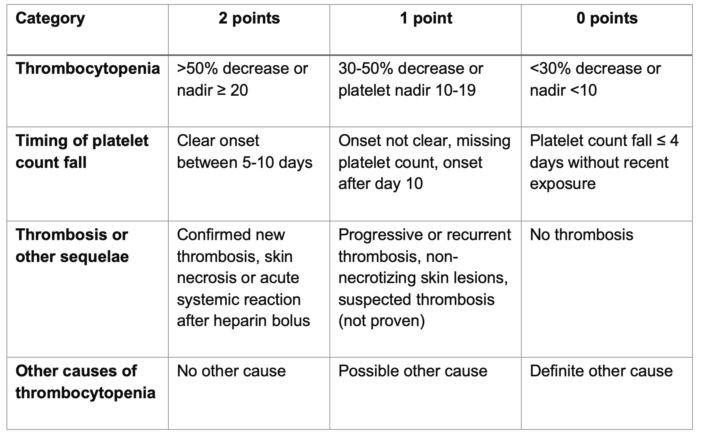
The 4Ts score is the sum of the values for each category. Scores 1-3, 4-5, and 6-8 correspond to a low, intermediate, and high probability of HIT, respectively.
Table 1. 4Ts score for HIT. Adapted from Cuker A, et al. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood. 2012; 120(20):4160-7.
Treatment
Management of HIT involves immediate discontinuation of heparin and initiation of alternative anticoagulation to prevent thrombotic complications.
1. Discontinuation of Heparin
- Heparin should be discontinued promptly upon suspicion of HIT to prevent further platelet activation and thrombosis.
- This includes all forms of heparin administration, including intravenous or subcutaneous unfractionated heparin and LMWH.
2. Initiation of Alternative Anticoagulation
- Direct thrombin inhibitors (DTIs), such as argatroban, bivalirudin, and lepirudin, are the mainstay of treatment for HIT.
- These agents directly inhibit thrombin, bypassing the need for heparin and preventing further thrombus formation.
- The choice of DTI and dosing regimen depends on factors such as renal function, concurrent medications, and the presence of thrombotic complications.
3. Monitoring and Adjustment
- Close monitoring of anticoagulation parameters, including activated partial thromboplastin time (aPTT) or activated clotting time (ACT), is necessary to ensure therapeutic anticoagulation with DTIs.
- Dosing adjustments may be required based on renal function, hepatic function, and the presence of bleeding or thrombotic complications.
4. Thrombosis Management
- Patients with HIT-associated thrombosis may require additional interventions, such as thrombectomy or thrombolysis, depending on the location and severity of thrombotic events.
- Consultation with hematology and critical care specialists is recommended for further management guidance.
HIT and Cardiopulmonary Bypass
Unfractionated heparin is the preferred anticoagulant for cardi0pulmonary bypass (CPB), but patients with HIT require special considerations and management.4
- Review the patient’s history and confirm the HIT diagnosis with appropriate laboratory tests. Given that HIT antibodies are transient, patients with a history of HIT more than 100 days prior should have serologic testing. If the platelet count has recovered and serologic testing is negative, patients can be re-exposed to heparin for CPB.
- Consult with the hospital protocol or hematology service for alternative anticoagulation strategies. Although the direct thrombin inhibitor bivalirudin is not FDA-approved for CPB, it has been shown to be a feasible alternative to heparin without significantly increased mortality or morbidity.
- Ensure no heparin products are in the CPB circuit or administered during the procedure. Bivalirudin dosing typically involves an initial loading dose followed by a maintenance infusion. The CPB pump prime will also contain bivalirudin. Dosing adjustments should be made for patients with renal impairment.
- Monitoring is essential during CPB to adjust the dosing of maintenance infusion. The activated clotting time (ACT) point-of-care test tends to be less precise at high concentrations of bivalirudin, and therefore, an ecarin clotting time (ECT) point-of-care test is recommended. However, ECT is not widely available, and ACT can be used as a safe alternative.
- Other considerations with bivalirudin include:
- Avoiding blood stasis during CPB, as local metabolism of bivalirudin can continue, leading to thrombus formation even with adequate systemic levels.
- Avoiding hypothermia after separation from CPB, as hypothermia reduces the proteolysis of bivalirudin and can contribute to coagulopathy.
References
- Ahmed I, Majeed A, Powell R. Heparin-induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83(983):575-82. PubMed
- Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012; 141, e495S–e530S. PubMed
- Salter BS, Weiner MM, Trinh MA, et al. Heparin-induced thrombocytopenia: A comprehensive clinical review. J Am Coll Cardiol. 2016;67(21):2519-32. PubMed
- Warkentin T E, Levine M N, Hirsh J.et al. Heparin‐induced thrombocytopenia in patients treated with low‐molecular‐weight heparin or unfractionated heparin. N Engl J Med. 1995;332(20):1330-5. PubMed
- Dwivedi R, Pomin VH. Marine antithrombotics. Mar Drugs. 2020;18(10): 514. PubMed
- Cuker A, Gimotty PA, Crowther MA, et al. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood. 2012; 120(20):4160-7. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.