Copy link
Drug Interactions
Last updated: 10/02/2024
Key Points
- There are two main types of drug interactions: pharmacokinetic (PK) and pharmacodynamic (PD).
- Our patient population is becoming increasingly more complex with a high incidence of polypharmacy and, therefore, an increased risk of drug interactions.
Introduction
- A drug-drug interaction occurs when the combination alters the effect of both or either drug on pharmacokinetic or pharmacodynamic parameters. This differs from an adverse drug reaction, which is a harmful reaction to a medication given at the appropriate dose.
- PK relates to how the drug is absorbed, distributed, metabolized, and eliminated from the body. A PK drug interaction will alter one of the following pathways:
- Absorption: the transfer from the site of administration to general circulation (this step is mostly eliminated with IV administration)
- Distribution: drug delivery from circulation into different organs
- Metabolism: in vivo chemical modification of the drug into active and/or inactive metabolites for elimination
- Elimination: the removal of the drug from the body through metabolism and excretion
- An example of a PK interaction is midazolam and propofol’s CYP450 interaction, which increases propofol’s duration of action.
- PD relates directly to a drug’s effect on its target site.1 Therefore, PD drug interactions occur when another drug’s effect is altered by the combination of another drug.
- PD interactions can be categorized as additive, synergistic (supra-additive), and antagonistic.
- An example of a PD interaction is a benzodiazepine and opioid combination potentiating the respiratory depressant effect.
Common Drug Interactions2
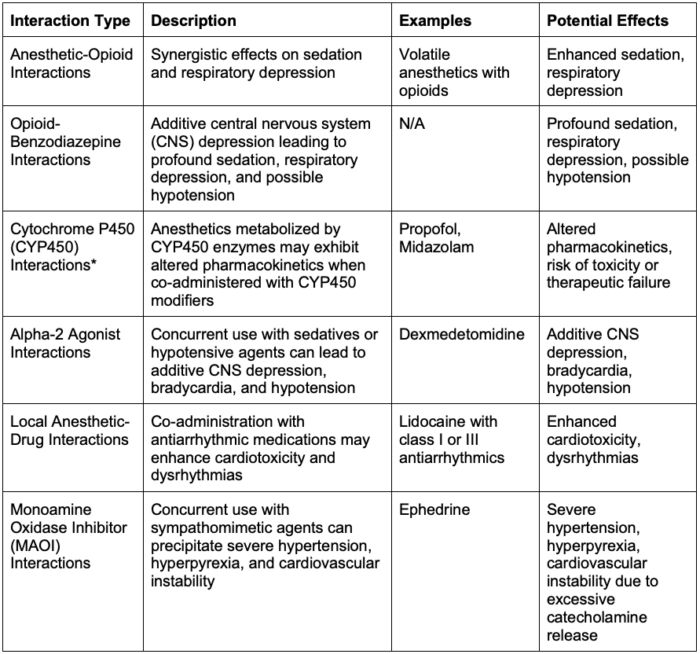
Table 1. Common anesthetic drug interactions
*CYP450 Inducers
- Phenytoin, carbamazepine, rifampin, and several chemotherapy agents induce hepatic CYP450 enzymes, thereby increasing enzymatic metabolic activity.
- This may result in the need for increased doses of both nondepolarizing neuromuscular blocking agents and propofol due to increased hepatic clearance.
- Propofol is a CYP450 3A4 inhibitor and can, therefore, prolong the duration of action of medications that are metabolized through this pathway (i.e., midazolam).
- It is also metabolized by the CYP450 2B6 enzyme, so inhibitors of this pathway, such as clopidogrel, can prolong its effect. In contrast, inducers such as phenytoin and carbamazepine can accelerate propofol’s metabolism and clearance.3
Specific Anesthetic Drug Interactions
- Vasoplegic syndrome and renin-angiotensin system antagonists (RAAS)4
- Vasoplegic syndrome is defined as severe hypotension refractory to catecholamine therapy with all other identifiable causes excluded.
- Vasoplegic syndrome occurs most commonly in cardiac surgery (8-10% of patients) and increases up to 50% in patients taking RAAS system agents.
- The proposed mechanism begins with an increased reliance on the RAAS system under general anesthesia to support blood pressure; therefore, inhibitors of this system prevent an appropriate physiologic response to hypotension.
- Treatment:
- Stopping the agent 24 hours preoperatively or skipping the morning dose if taken twice daily.
- Some long-acting agents need to be stopped more than 24 hours preoperatively.
- Vasopressors: vasopressin may be the pressor of choice if the patient is not responding to other usual agents.
- Methylene blue interferes with nitric oxide-cyclic guanosine monophosphate smooth muscle relaxant effect. It is contraindicated in renal impairment as it may cause hemolysis and methemoglobinemia and interfere with pulse and cerebral oximetry readings.
- Neuromuscular blocking agent (NMBA) interactions: See OA summary on the effects of drugs on neuromuscular blockade. Link
- Potentiation/prolongation of neuromuscular blockade:5
- Select antibiotics (e.g., aminoglycosides, tetracyclines, clindamycin) can inhibit the release of acetylcholine (Ach) or desensitize postjunctional nicotinic ACh receptors to Ach.
- Acute anticonvulsant therapy (e.g., phenytoin)
- Inhaled anesthetics inhibit nicotinic Ach receptors and potentiate neuromuscular blockade of nondepolarizing NMBAs.
- Potentiation effect in decreasing order: desflurane, sevoflurane, isoflurane, nitrous oxide
- Lithium: structurally like cations that activate K+ channels, inhibiting neuromuscular transmission
- Magnesium: decreases muscle membrane excitability by reducing prejunctional ACh release
- Resistance to NMBA
- Chronic anticonvulsant therapy has been shown to shorten the duration of action and reduce the potency of nondepolarizing NMBAs.
- The proposed mechanism is due to the induction of hepatic enzymes by anticonvulsants which results in increased metabolism and hepatic clearance.
- Secondarily, agents such as phenytoin and carbamazepine may alter the neuromuscular junction and reduce the potency of NMBA.
- Statins with succinylcholine
- The mechanism is unclear but has been shown to potentially worsen myalgias due to increased fasciculations with succinylcholine.6
- Naltrexone and opioids5
- Naltrexone is a competitive opioid receptor antagonist with highest affinity for mu receptors.
- It requires high enough doses of opioids to overcome competitive antagonism for effective mu receptor activation and associated analgesia.
- Alcohol and anesthetics
- Chronic users have induction of hepatic enzymes and, therefore, increased metabolism and elimination of many common anesthetic agents (i.e., propofol)
- Sugammadex/hormone interactions7
- Sugammadex is a steroid binder that can potentially bind progestins in hormonal contraceptives and reduce their efficacy.
- Binding studies suggest that sugammadex may bind to progestogen, and receiving sugammadex on the same day as a hormonal contraceptive should be viewed as equivalent to missing contraceptive doses on those days. Sugammadex at a 4 mg/kg dose is expected to decrease progestin exposure by about 34%.
- Potentiation/prolongation of neuromuscular blockade:5
Prevention and Treatment for Drug Interactions
- Prevention
- Comprehensive understanding of drug interactions
- Appropriate discontinuation of selected drugs preoperatively based on clinical situation and patient factors.
- Appropriate monitoring for known interactions
- Treatment
- Avoidance/discontinuation of offending drugs preoperatively where possible.
- Understand the half-life of a drug for appropriate preoperative stop date – 5½ lives are sufficient for near full clearance.
- Consider the withdrawal effects of stopping medications
- Understand the pharmacokinetic and pharmacodynamic effects of each drug and how anesthetics may require dose adjustment
- Tailor anesthetics to account for medications that are not discontinued appropriately or continued appropriately
References
- Tafur-Betancourt L. El mundo oculto de las interacciones farmacológicas en anestesia. Rev Colomb Anestesiol. 2017; 45:216–223. Link
- Bravenec B. General anesthesia: Intravenous induction agents. In: Post T, ed. UpToDate, 2023. Accessed Sept 6, 2024. Link
- Drug-drug interactions (DDI). Anesthesia Patient Safety Foundation. Accessed September 6, 2024. Link
- Shear T, Greenberg S. Vasoplegic syndrome and renin-angiotensin system antagonists. Anesthesia Patient Safety Foundation Newsletter. 2012. 27(1). Accessed September 6, 2024. Link
- Renew JR. Clinical use of neuromuscular blocking agents in anesthesia. In: Post T, ed. UpToDate, 2023. Accessed September 6, 2024. Link
- Elvir-Lazo OL, White PF, Cruz Eng H, et al. Impact of chronic medications in the perioperative period -anesthetic implications (Part II). Postgrad Med. 2021;133(8):920-38.
- Dwan, RL, Raymond, BL, Richardson, MG. Unanticipated consequences of switching to sugammadex: Anesthesia provider survey on the hormone contraceptive drug interaction. Anesth Analg. 2021;133(4): 958-66. PubMed
Other References
- US Food and Drug Administration. Prescribing Information: Bridion (sugammadex). Vol Reference ID: 3860969. December 2015. Accessed February 24, 2021. Link
- Struys, MF. Drug interactions in anaesthetic practice, in Hardman, JG, Hopkins, PM, and Struys, MF (eds), Oxford Textbook of Anaesthesia, Oxford Textbook (Oxford, 2017; online edn, Oxford Academic, 1 Apr. 2017), accessed 21 Mar. 2024. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.