Copy link
Succinylcholine
Last updated: 04/24/2024
Key Points
- Succinylcholine is a short-acting, depolarizing neuromuscular blocking agent (NMBA) that is commonly used for rapid-sequence induction and to treat laryngospasm.
- Onset of action typically occurs within 30 to 60 seconds after intravenous (IV) administration and 2-3 minutes with intramuscular (IM) use.
- Metabolism occurs via breakdown by pseudocholinesterase as the drug diffuses away from the neuromuscular junction.
- Succinylcholine administration has numerous side effects, including, but not limited to, myalgias, hyperkalemia, malignant hyperthermia, and a transient increase in intraocular and intracranial pressure.
Structure/Physicochemical Characteristics
- Succinylcholine is produced in the chloride form as succinylcholine chloride or suxamethonium chloride.
- Succinylcholine is composed of two acetylcholine (ACh) molecules linked together through the acetate methyl groups (Figure 1).
- 2,2’-[(1,4-dioxo-1,4-butanediyl)bis (oxo)]bis[N,N,N,-trimethylethanaminium] dichloride)1
- Succinylcholine acts as an ACh agonist at both postsynaptic and extra-junctional ACh receptors.2-4
- It has low lipid solubility with a small volume of distribution.
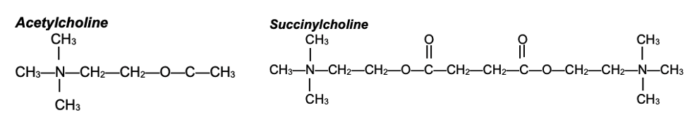
Figure 1. Structures of ACh and succinylcholine. Succinylcholine consists of two ACh molecules linked by an acetate methyl group.
Mechanism of Action2-5
- Succinylcholine binds to the ɑ subunits of the nicotinic acetylcholine receptor.
- Once bound to the receptor, depolarization of the postjunctional membrane occurs within 30 to 60 seconds after IV administration, leading to muscle fasciculation followed by flaccid paralysis.
- Sodium channels are inactivated, and neuromuscular transmission is blocked. This results in desensitization of the nicotinic Ach receptor (i.e., phase I or accommodation block).
Metabolism and Excretion
- The duration of action of succinylcholine is 5-7 minutes after IV administration.
- Only a small portion of the injected drug (5-10%) ever reaches the neuromuscular junction.
- There is little to no plasma cholinesterase at the neuromuscular junction, so the action of succinylcholine is terminated by diffusion from the endplate to the extracellular fluid where it is then metabolized.
- Metabolism occurs via break down by pseudocholinesterase (plasma cholinesterase) into succinylmonocholine, which has very weak neuromuscular blocking activity.
- Succinylmonocholine is then hydrolyzed into succinic acid and choline.
- Pseudocholinesterase is synthesized in the liver. It is responsible for the metabolism of succinylcholine, mivacurium, and ester local anesthetics.
Change in the Effectiveness of Succinylcholine
Upregulation of ACh Receptor Sites
- Increased sensitivity to depolarizing NMBAs and resistance to nondepolarizing NMBAs:
- Upper and lower motor neuron lesions
- Burns
- Severe infection
- Prolonged use of neuromuscular blocking agents
- Muscle trauma
- Cerebral palsy
- Chronic use of anticonvulsant agents
Downregulation of ACh Receptor Sites6
- Resistance to depolarizing NMBAs and increased sensitivity to nondepolarizing NMBAs
- Myasthenia gravis
- Organophosphate poisoning
- Exercise conditioning
Activity Changes with Pseudocholinesterase
- Decreased pseudocholinesterase activity or levels
- Liver disease
- Uremia
- Malnutrition
- Hypothyroid
- Third trimester of pregnancy
- Newborns
- Increased pseudocholinesterase activity or levels
- Obesity
- Alcoholism
- Hyperthyroid
- Psoriasis
- Electroconvulsive therapy
- Drugs that decrease pseudocholinesterase activity
- Echothiophate
- Neostigmine
- Cyclophosphamide
- Metoclopramide
- Esmolol
- Oral contraceptives
Pseudocholinesterase Deficiency
- Pseudocholinesterase deficiency results in prolonged activity of succinylcholine leading to longer paralysis and often requires mechanical ventilation.
- Patients will need to be sedated to prevent patient awareness of paralysis.
Dosage
- The IV dose of succinylcholine is 0.5-0.75 mg/kg (up to 1 mg/kg) for adults with an onset time of 30 to 60 seconds.
- In obese patients, succinylcholine should be dosed based on total body weight, secondary to an increased volume of distribution and increased pseudocholinesterase activity.
- The intramuscular dose of succinylcholine is 4-5 mg/kg.
- Infants have an increased total body water and extracellular fluid compartment compared to adults, creating a larger volume of distribution and a higher dose requirement of 2 mg/kg.
- An IV infusion can be used for short cases that need deep muscle relaxation. The dose in adults is 2.5-4.3 mg/minute.7
- Succinylcholine should be refrigerated; once removed it should be used within 14 days.
Clinical Use
- Muscle relaxation for rapid-sequence induction
- Muscle relaxation for easier masking and intubation
- To treat laryngospasm
Side Effects3-5
Hyperkalemia
- Normal muscle releases enough potassium during succinylcholine-induced depolarization to raise serum potassium by ~ 0.5 mEq/L.
- Upregulation of extra junctional receptors can cause a much higher increase in potassium levels, causing possible symptoms of severe hyperkalemia, including cardiac arrest.
- Conditions raising susceptibility to succinylcholine-induced hyperkalemia:
- Burn injuries
- Spinal cord injuries
- Stroke with residual symptoms
- Guillain Barre syndrome
- Multiple sclerosis
- Muscular dystrophy
- Amyotrophic lateral sclerosis
- Parkinson disease
- Prolonged immobilization
- Myopathies
- Massive trauma
- Tetanus
- Ruptured cerebral aneurysm
- Hemorrhagic shock with metabolic acidosis
- See the OA summary on the effects of medical conditions on NMBAs for more details Link
- The risk of hyperkalemia after burn injuries, spinal cord injuries, and stroke peaks at 7-10 days. This is due to the upregulation of extra-junctional acetylcholine receptors. There is minimal risk within the first two days of injury. Upregulation of Ach receptors lasts up to 2 years; during this time, succinylcholine should be avoided due to concern for severe hyperkalemia.
- Renal failure itself does not increase the risk of succinylcholine administration as long as the serum potassium is below 5 mEq/L.
Myalgia
- Myalgias may occur secondary to unsynchronized muscle fasciculations.
- This may be minimized by administering a “precurarization” dose of a nondepolarizing neuromuscular blocker (35% of ED95) before succinylcholine administration.
- Nonsteroidal anti-inflammatory drugs, lidocaine, and benzodiazepines may minimize the occurrence of myalgias.
Masseter Muscle Rigidity
- After succinylcholine administration, increased tone of the masseter muscle may be observed, resulting in limited mouth opening.
Malignant Hyperthermia
- Succinylcholine is a triggering agent for malignant hyperthermia.
Cardiac Arrhythmias8
- Succinylcholine has a similar appearance to acetylcholine and may act upon the sinus node, leading to bradycardia or tachycardia, depending on the patient’s age.
- A second dose within 5 minutes of the initial dose can cause severe bradycardia, dysrhythmias, or asystole. This usually resolves within one minute.
- Bradycardia can occur in some patients, especially children.
- Using a continuous infusion has a higher risk of causing bradycardia.
Increased Intraocular Pressure
- Transiently increased intraocular pressure may occur, lasting 5 to 10 minutes, due to extraocular muscle contraction, which leads to decreased aqueous outflow.
- While succinylcholine is associated with a transient increase in intraocular pressure, there are no published reports of extrusion of ocular contents when it is administered along with an adequate dose of an anesthetic induction agent.
Increased Intracranial Pressure
- There is a mild transient increase in intracranial pressure.
- Succinylcholine is not contraindicated in patients with an intracranial mass or increased intracranial pressure.
Increased Intragastric Pressure
- Succinylcholine increases intragastric pressure due to abdominal muscle contraction.
- Likewise, lower esophageal sphincter pressure also increases, which results in a maintained gastroesophageal barrier, preventing reflux.
Histamine Release
- Histamine release may occur after succinylcholine administration.
- A rash on the patient’s chest can be seen in up to 50% of patients.
Phase II Blockade
- If repeated doses or a prolonged infusion of succinylcholine is given, a phase II block can develop, which is characterized by a fade in the train-of-four response, much like a competitive block from a nondepolarizing NMBA.2
References
- Alvarellos, ML, McDonagh EM, Patel S, et al. PharmGKB summary: Succinylcholine pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet Genomics. 2015; 25(12): 622-30. PubMed
- Martyn JA, Fagerlund MJ. Neuromuscular physiology and pharmacology. In: Gropper M, Cohen NH, Miller RD, et al.(eds) Miller’s Anesthesia. Philadelphia, PA. 9th edition. Elsevier; 2019:333-353.
- Appiah-Ankam J, Hunter JM. Pharmacology of neuromuscular blocking agents. Continuing Education in Anaesthesia Critical Care & Pain. 2004; 4(1):2-7. Link
- Brull SJ, Meistelman C. Pharmacology of neuromuscular blocking drugs. In: Gropper MA, Cohen NH, Miller RD, et al.(eds) Miller’s Anesthesia. Philadelphia, PA. 9th edition. Elsevier; 2019: 792-831.
- Weigel WA, Grant SA, Thilen SR. Neuromuscular blocking drugs. In: Barash, Cullen, and Stoelting’s Clinical Anesthesia. 9th edition, Philadelphia, PA. Wolters Kluwer. 2023.
- Amundsen HB, Sørensen MK, Gätke MR. Succinylcholine resistance. Br J Anaesth. 2015;115(6): 818-821. PubMed
- Succinylcholine package insert. Accessed April 22nd, 2024. Link
- Vanneman M, Madhok J, Weimer JM. et al. Perioperative implications of the 2020 American Heart Association scientific statement on drug-induced arrythmias-A focused review. J Cardiothor Vasc Anesth. 2022;36(4):952-61. PubMed
- Faulk DJ. Neuromuscular blockade and anticholinesterases. In: Chu LF, Traynor AJ, Kurup V. Manual of Clinical Anesthesiology. Philadelphia, PA. 2nd edition. Wolters Kluwer; 2020: 223-231.
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.