Copy link
Down Syndrome
Last updated: 12/03/2024
Key Points
- Down syndrome is the most common major chromosomal abnormality, and these patients frequently have significant multiorgan system comorbidities.
- Patients with Down syndrome present with facial dysmorphia, a large tongue, obstructive and central sleep apnea, and atlantoaxial instability. Careful consideration should be taken during airway management and anesthetic planning.
- Anesthetic concerns include a proclivity for bradycardia, difficulty with IV access, cardiac defects, and sensitivity to opioids and atropine.
Introduction
- Down syndrome (DS) is the most common trisomy or major chromosomal abnormality, with an occurrence of 1 in 700 live births.1
- DS is most commonly caused by the presence of an additional copy of chromosome 21. Certain rare genetic variations with similar phenotypic outcomes may be caused by chromosomal translocation events or genetic mosaicism.1
- John Down, an English physician, first described the common patient features in 1866, but its association with trisomy 21 was not established until nearly 100 years later.2
- The incidence of DS increases with maternal age, with the risk increasing exponentially starting around ages 35-40.2
- The life expectancy of DS patients has quadrupled in the last 50 years, with an average life expectancy of 60 years in the United States.1
- Cardiovascular and respiratory problems are the two most common causes of death. Trisomy 21 increases the risk of developing leukemias and Alzheimer’s disease, which may further impact functional status and quality of life.1,3
- Intellectual disability is common, although mild and moderate disability is more frequent than severe.1
Diagnosis
- Prenatal diagnosis may be made via chorionic villus sampling or amniocentesis, while other newborns may present with undiagnosed DS.1
- Conditions detected antenatally may include congenital cardiac anomalies, duodenal atresia, and tracheoesophageal fistula (TEF).3,4
- Typical craniofacial features in patients with DS of all ethnicities include a short neck with excess skin folds posteriorly, flattened nose and facial profile, small head, low-set ears and mouth, upward slanting palpebral fissures with epicanthal folds, and macroglossia.3,4
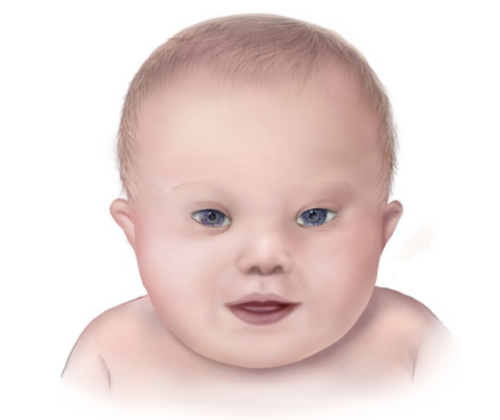
Figure 1. Typical facial features of a child with Down syndrome. Note the flattened nose, low-set ears, short neck, upward-slanting eyes, and large tongue. Source: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities, Public domain, via Wikimedia Commons. Link
Comorbidities
Airway Abnormalities and Respiratory Disorders
- Changes to airway anatomy include a flat nasal bone, midface hypoplasia, a large tongue and a short palate, and excess tonsillar tissue.3
- As many as 65% of DS patients will exhibit some form of sleep-disordered breathing, including obstructive sleep apnea. This number is likely even higher, given the technical difficulties associated with conducting sleep studies in this population.3
- Joint laxity is common at the atlantoaxial and atlantooccipital joints, leading to potential cervical spine instability (Figure 2). As many as 30% of children will have cervical spine radiographs consistent with increased movement at this junction. This is also exacerbated by generalized hypotonia seen in these patients.3
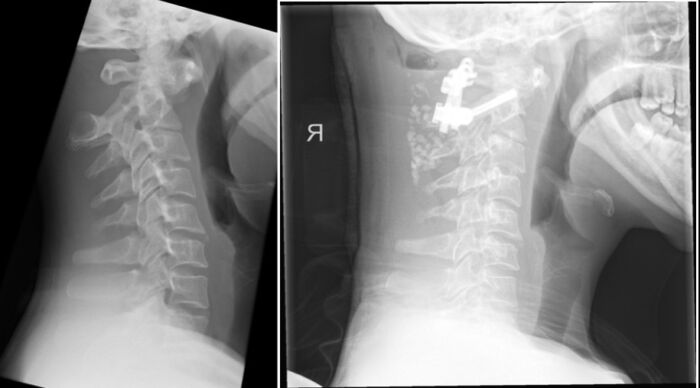
Figure 2. Severe anterior atlantoaxial subluxation in a patient with Down syndrome (left). Postoperative study, with interval C1 / C2 fusion (right). Case courtesy of Sze Wing Angel Pang, Radiopaedia.org, rID: 155197. Link
- Routine preoperative cervical spine X-rays are not recommended for asymptomatic patients and are not required in the absence of clinical concerns.4,5
- A cervical spine workup is indicated if a patient presents with new neurologic deficits, such as weakness of an extremity, pain in the neck, or gait disturbance. For urgent procedures with new-onset neurologic deficits, cervical spine precautions are indicated.3
Congenital Heart Disease
- Approximately half of all children with DS will exhibit at least one cardiac defect, and cardiac workup is highly advisable.3,4 The American Association of Pediatrics (AAP) recommends that an echocardiogram and evaluation by a pediatric cardiologist be performed by 6 months of age.5 The AAP does not make specific recommendations on how frequently to monitor for cardiac defects, and the decision to obtain new imaging should be made on a case-by-case basis.
- The most common cardiac defects are endocardial cushion defects (43% of lesions), followed by ventricular septal defects (32%) and atrial septal defects (10%).3
- The high likelihood of cardiac lesions and imbalanced flow presents an elevated risk for the development of pulmonary hypertension and resulting complications, such as hypoxia. This is additionally compounded by the independent risk factors of airway obstruction and sleep-disordered breathing, which also predispose the patient to pulmonary hypertension.3,4,6
Neurologic Disorders
- An estimated 5-13% of patients with DS will experience seizures as children, especially before the age of 1 year. By the age of 30, 40% of patients will experience seizures (typically tonic-clonic or myoclonic).2
- Fifty to 60% of patients will develop dementia by the age of 60.2
- Nearly all patients will have at least a mild learning disability, and moderate disability is frequently seen.2
Ocular and Otorhinolaryngologic Disorders
- Common ocular manifestations include cataracts, strabismus, and amblyopia.1 Eye exams are recommended within 6 months of life to screen for abnormalities.2
- Hearing loss may result from chronic otitis media.1
- The auditory canals tend to be narrow and thus more subject to cerumen impaction and its consequences.2
Musculoskeletal Disorders
- Eighty percent of DS patients will exhibit hypotonia.1
- Ligamentous laxity predisposes to joint dislocation;2 see airway section for cervical spine considerations
Endocrine Disorders
- Hypothyroidism is common, and diabetes tends to develop later in life.1,2
- Various abnormalities in sexual development are common, including primary hypogonadism, with associated delayed puberty and infertility.2
Gastrointestinal Disorders
- Gastrointestinal comorbidities occur in 10-12% of DS patients. Frequently associated conditions include duodenal atresia, TEF, and Hirschsprung’s disease.3
- The double bubble sign is a classic ultrasound or x-ray finding demonstrating two distinct “bubbles” of air in the stomach and proximal duodenum, resulting from duodenal atresia (Figure 3).2
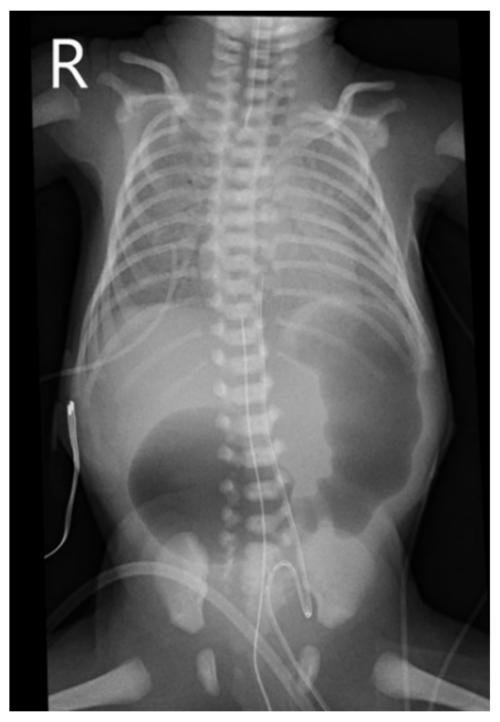
Figure 3. Marked gaseous distension of the proximal duodenum and stomach, leading to two large “bubbles” of air on plain radiographs. Source: Case courtesy of Alexandra Stanislavsky, Radiopaedia.org, rID 47635. Link
- In addition to structural defects, DS patients are prone to gastroesophageal reflux and chronic constipation.2
Hematologic Abnormalities
- Newborns with DS often have neutrophilia, thrombocytopenia, and polycythemia, with frequencies of 80%, 66%, and 34%, respectively. This is usually mild and resolves within 3 weeks of life.2,4
- The risk of developing leukemia is 10 to 15-fold higher than that of nonsyndromic counterparts, most commonly due to mutations in the JK2 gene.2,4
Preoperative Considerations
- Mask induction is acceptable for most DS patients weighing less than 50 kg. Vascular access in the preoperative area should be considered in the context of patient size, previous anesthetic complications, previous difficulty with IV placement, suspected difficult mask induction, and cardiac comorbidities.
- Many DS patients may benefit from preoperative anxiolysis, like their nonsyndromic pediatric counterparts. Oral midazolam is a popular choice for those patients amenable to drinking it. Intellectual impairment may cover a wide spectrum; caregivers can help assess baseline functional status and optimize compliance with preoperative medications.3
- Intramuscular ketamine administration may facilitate transporting a patient to the operating room, especially for uncooperative older DS children and adolescents. One possible risk includes increased oropharyngeal secretions associated with ketamine usage; however, this may be offset by the xerostomia associated with the administration of dexmedetomidine or glycopyrrolate once intravascular access is obtained.
- Targeted questions to the patient and/or caregivers can help assess for snoring, daytime sleepiness, restless sleep, and pauses in breathing. Patients with DS are often overweight or obese, which may compound difficulties with sleep-disordered breathing.1 A thorough physical exam assessing the airway, cardiac, and pulmonary systems must be performed.
- Patients with DS are scheduled for a wide range of diagnostic and therapeutic procedures (Table 1).
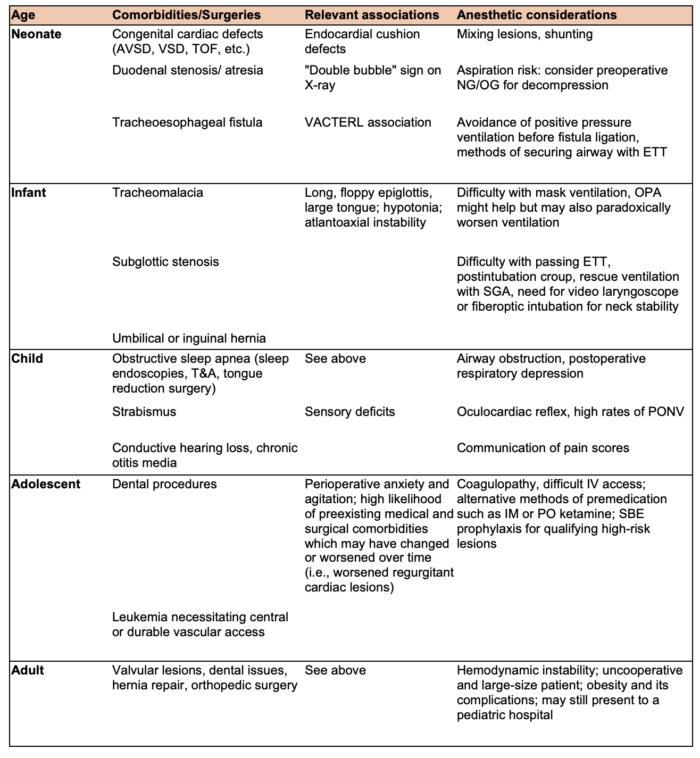
Table 1. Common surgical procedures and anesthetic considerations by age. AVSD: atrioventricular septal defect, VSD: ventricular septal defect, TOF: Tetralogy of Fallot, NG/OG: nasogastric/orogastric, ETT: endotracheal tube, OPA: oropharyngeal airway, SGA: supraglottic airway, T&A: tonsillectomy and adenoidectomy, PONV: postoperative nausea and vomiting, SBE: subacute bacterial endocarditis.
Intraoperative Considerations
- Although many patients with DS exhibit cognitive delay, they may still have sufficient social competence and emotional self-regulation to cooperate to a limited extent with the anesthesia induction process.3
- Profound bradycardia following sevoflurane induction occurs with significantly higher frequency in children with DS compared to nonsyndromic children (57% vs 12% in one study). Consequences of bradycardia may include hypotension. Profound bradycardia may occur suddenly and is unlikely to be related to noxious stimuli.7 Autonomic mechanisms, such as parasympathetic excess or sympathetic failure, have been postulated as the likely cause of this bradycardia.8
- Bradycardia is usually but not always responsive to anticholinergic rescue medications such as glycopyrrolate and atropine. Rapid administration of epinephrine should be considered if bradycardia remains uncorrected after administration of anticholinergics, preferably intravenously, if access has been achieved. Decreasing the concentration of inhaled sevoflurane is also frequently effective and is therefore recommended.7
- Using a video laryngoscope reduces the amount of head extension required, thus reducing the risk of C1-C2 subluxation, and may be the most appropriate first-line technique for intubation. A fiberoptic bronchoscope may be a useful airway adjunct for management or as a primary method of placing an endotracheal tube (ETT).9
- An oropharyngeal airway and gentle jaw thrust are often required to maintain a patent airway due to a large tongue, small mouth, midface hypoplasia, and baseline hypotonia typical of DS patients.3 The large tongue, especially when combined with hypertrophied lingual tonsils, may cause unanticipated difficulty with ventilation.4,6
- When endotracheal intubation is indicated, subglottic stenosis (relatively common in this population) may require using an ETT one or even two sizes smaller than expected for smooth passage into the trachea. Subglottic stenosis is usually, though not always, associated with a history of prior intubations.3
- A leak test should be carefully performed when the circuit is pressurized to 20 cm H2O to ensure a satisfactory match between the ETT and patient size, reducing the risk of pressure injury around the endotracheal balloon while ensuring the ability to ventilate.3,6
- A supraglottic airway can also be an effective ventilation device as a first-line modality or rescue option.3,6
Postoperative Considerations
- Patients with DS are at high risk of postoperative airway complications such as airway obstruction and postintubation croup; therefore, vigilance should remain high. Pulse oximetry and end-tidal CO2 monitoring should be available. PACU observation by skilled personnel is paramount.3,9
- If postintubation croup occurs, the most common time frame is 20-30 minutes after removal of the ETT. This may be treated with inhaled racemic epinephrine and IV steroids (such as dexamethasone).3,6
- DS patients are at increased risk of inadequate pain control in the postoperative period due to multiple factors. Larger doses of narcotics are usually withheld, given the predisposition towards airway obstruction. Additionally, DS patients are slower to express pain scores and less precise when localizing a painful stimulus compared to controls.10
- Blockade with local anesthetics, whether peripheral or neuraxial, are excellent adjuncts for pain management without the same risks of respiratory depression and should be considered with compatible procedures for postoperative pain management.3
- Depending on the severity of a patient’s comorbidities, additional postoperative monitoring in an ICU environment can be considered on a case-by-case basis. Concerns include hypercapnia, hypoxemia, postextubation croup, management of noninvasive and invasive ventilation, and cardiac lesions.9
References
- Ohio Department of Health. Down Syndrome Fact Sheet. Ohio Department of Health. Jun 2024. Accessed Oct 2024. Link
- Akhtar F, Bokhari SRA. Down syndrome. In: StatPearls (Internet). Treasure Island, FL. StatPearls Publishing; 2024. Accessed November 2024. Link
- Wittkugel EP, Samol, NB. Special Pediatric Disorders. In: Davis PJ, Cladis, FP. Smith’s Anesthesia for Infants and Children. 9th ed. Philadelphia, PA; Elsevier; 2017: 13301333.
- Lewanda AF, Matisoff A, Revenis M, et al. Preoperative evaluation and comprehensive risk assessment for children with Down syndrome. Paediatr Anaesth. 2016;26(4):356-62. PubMed
- Bull MJ. Health supervision for children with Down syndrome. Pediatrics. 2011; 128(2):393-406. PubMed
- Steward DJ. Anesthesia considerations in children with Down syndrome. Seminars in Anesthesia, Perioperative Medicine and Pain. 2006;25(3): 136-141. Link
- Roodman S, Bothwell M, Tobias JD. Bradycardia with sevoflurane induction in patients with trisomy 21. Paediatr Anaesth. 2003;13(6):538-40. PubMed
- Sinton JW, Cooper DS, Wiley S. Down syndrome and the autonomic nervous system, an educational review for the anesthesiologist. Paediatr Anaesth. 2022;32(5):609–616. PubMed
- Fiadjoe JE, Litman RS, Serber JF, Stricker PA, Cote CJ. The Pediatric Airway. In: Cote CJ, Lerman J, Anderson BJ . A Practice of Anesthesia for Infants and Children. 6th ed. Philadelphia, PA; Elsevier; 2019: 297-33.
- Hennequin M, Faulks D, Veyrune JL, Bourdiol P. Significance of oral health in persons with Down syndrome: a literature review. Dev Med Child Neurol. 1999;41(4):275-83. PubMed
Other References
- Ambardekar A. Trisomy 21. OA Pediatric Anesthesia Vodcast. 2018. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.