Copy link
Disseminated Intravascular Coagulation
Last updated: 02/02/2024
Key Points
- Disseminated intravascular coagulation (DIC) is a syndrome characterized by widespread activation of the coagulation system in response to an underlying pathology, commonly infection, malignancy, trauma, or obstetric complication.
- Widespread activation of the coagulation cascade and resulting deficiencies of coagulation factors cause both life-threatening hemorrhage and microvascular thrombosis.
- Treatment is primarily supportive and should focus on correcting the causal pathology.
Introduction
- DIC is a clinical syndrome characterized by widespread and uncontrolled activation of the coagulation system leading to consumption of platelets and coagulation factors, fibrin deposition in the vasculature, organ dysfunction, and life-threatening hemorrhage.1,2
- Numerous clinical conditions have been linked to the development of DIC including sepsis, trauma, malignancy, and obstetric complications including placental abruption and amniotic fluid embolism (Table 1).2

Table 1. Causes of DIC. Adapted from Papageorgiou C, et al. Disseminated intravascular coagulation: An update on pathogenesis, diagnosis, and therapeutic strategies. Clin Appl Thromb Hemost. 2018;24(9_suppl):8S-28S.2 CC BY NC 4.0 PubMed
- The clinical presentation of DIC is highly variable based on the balance of microvascular thrombosis and hemorrhage from consumption of coagulation factors, platelets, and inhibitors.1 Most common presentations include bleeding, renal dysfunction, lung injury, and central nervous system dysfunction.
- The pathophysiology of DIC differs by the clinical condition. Broadly, alterations in endothelial function and subsequent decrease in anticoagulant activity can lead to increased activation of the coagulation cascade. Tissue factor overexpression is a universal agent implicated in the development of DIC.
Pathophysiology
- Molecular pathways involved in the development of DIC are complex and continue to be a subject of ongoing research. In this section we will focus on the major pathophysiologic pathways by which various clinical conditions lead to microvascular thrombosis and hemorrhage seen in DIC.1,2
Increased Expression of Tissue Factor
- Increased levels of tissue factor (TF) expression is the predominant trigger for overactivation of the coagulation cascade in DIC (Figure 1).
- TF is a procoagulant membrane protein that initiates in vivo hemostasis. Binding of TF to activated factor VII (FVIIa) forms an enzymatic complex which activates factor X, leading to the production of thrombin from prothrombin. Thrombin then leads to the cleavage of fibrinogen to fibrin. Fibrin cross-links lead to the formation of a stable clot.
- The cause of TF overexpression differs based on the etiology.1
- Amniotic fluid embolism, placental rupture, and trauma lead to the direct exposure of circulating blood to TF.
- In sepsis and malignancy, TF is expressed on circulating monocytes and tumor-derived cells respectively.
Platelet Overactivation
- Epithelial dysfunction and thrombin expression both lead to the overactivation of circulating platelets.
- Increased levels of von Willebrand Factor and decreased levels of ADAMS13 lead to further platelet activation.
- Platelet aggregation within the microcirculation leads to microthrombus formation and end-organ dysfunction.1
Altered Fibrinolysis
- The generation of fibrin and tissue hypoxia cause the release of tissue plasminogen activator (tPA) from the vascular endothelium resulting in enhanced activation of plasminogen to plasmin, the active enzyme that hydrolyzes cross-linked fibrin.
- Plasminogen activator inhibitor-1 (PAI-1) released from vascular epithelium inhibits tPA and effectively regulates the initial phase of fibrinolysis.
- α2-plasmin inhibitor is an irreversible inhibitor of plasmin that circulates at levels that neutralize plasmin under normal conditions.
- A global inflammatory response such as that seen in severe sepsis leads to PAI-1 release from vascular epithelium as well as activated platelets. Increased levels of PAI-1 produce a hypofibrinolytic state due to the enhanced inhibition of tPA.
- Malignancy often produces a hyper-fibrinolytic state due to plasminogen.
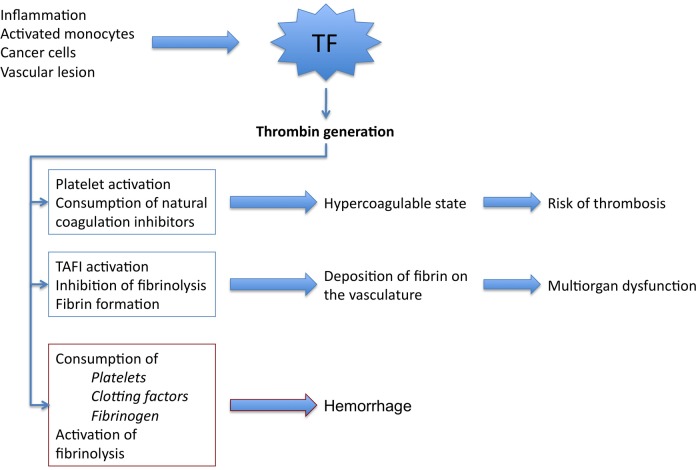
Figure 1. Pathophysiology of DIC. Source: Papageorgiou C, et al. Disseminated intravascular coagulation: An update on pathogenesis, diagnosis, and therapeutic strategies. Clin Appl Thromb Hemost. 2018;24(9_suppl):8S-28S.2 CC BY NC 4.0. PubMed
Diagnosis
- There is no single diagnostic test for DIC. The diagnosis is made by diagnostic scoring systems that account for laboratory testing, symptoms, and the presence of a clinical condition linked to DIC.1,2
- Laboratory testing indicative of DIC:
- Low or rapidly decreasing platelet count
- Prolonged activated partial thromboplastin time (aPTT) and prothrombin time (PT)
- Low fibrinogen
- Low antithrombin activity
- Elevated fibrin breakdown products including D-dimer
- Scoring systems have been developed by several professional societies, including the International Society on Thrombosis and Haemostasis (Table 2), the Japanese Society for Thrombosis and Hemostasis (Table 3), the Japanese Ministry Health and Welfare, the Japanese Association for Acute Medicine, the British Committee for Standards in Haematology, and the Italian Society of Thrombosis and Hemostasis.
- Utility of rotational thromboelastography has not been established but is common in the evaluation of trauma-associated coagulopathy and correlates well with established DIC scoring systems.
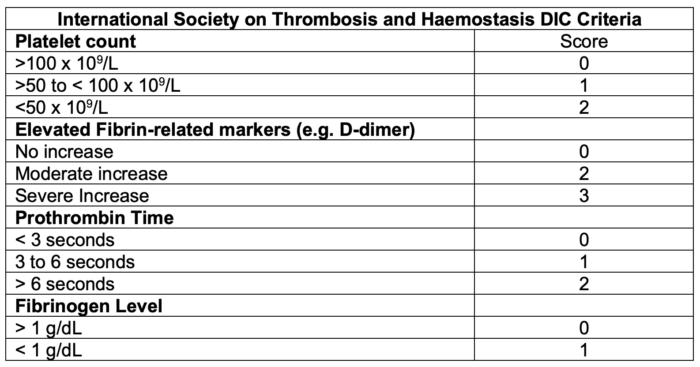
Table 2. DIC scoring system by the International Society on Thrombosis and Haemostasis.3 A score greater or equal to 5 is compatible with DIC.
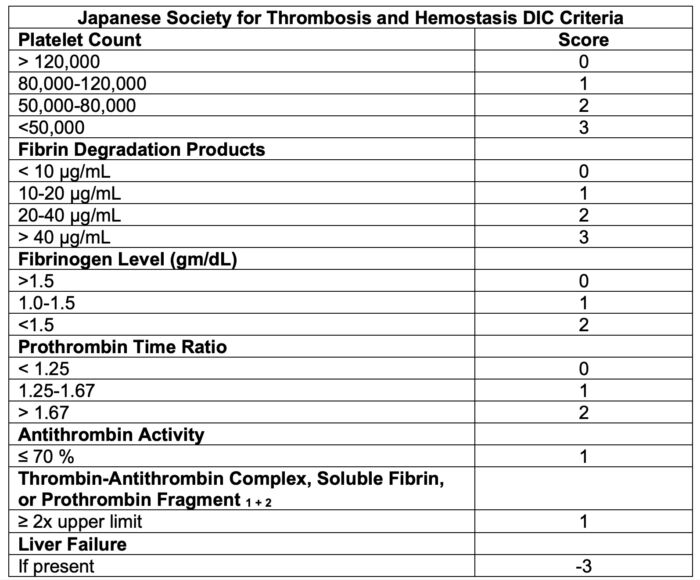
Table 3. DIC scoring system by the Japanese Society for Thrombosis and Hemostasis DIC Criteria.4 A score greater than or equal to 6 is compatible with DIC.
Clinical Presentation
Trauma
- Trauma-induced coagulopathy often presents itself as a hyperfibrinolytic type of DIC characterized by massive bleeding.
- Pathophysiology of DIC in trauma is the result of activation of coagulation and the resultant impairment of endogenous anticoagulants such as antithrombin and protein C. Activation of coagulation and release of endogenous tissue-plasminogen activator lead to increased fibrinolysis.
- The widespread activation of the coagulation system leads to a consumptive coagulopathy resulting in decreased levels of circulating coagulation factors and platelets.
Sepsis
- In sepsis, both the coagulation and fibrinolytic systems are altered due to extensive immune cell activation and endothelial damage. This often presents as a hypofibrinolytic state characterized by microvessel thrombosis and ischemic organ dysfunction.
- Neutrophil activation causes the release of deoxyribonucleic acid, histones, and other neutrophil granule proteins that are known to be prothrombotic.
- Alterations in endothelial production of tissue-type plasmin activator and plasminogen activation inhibitor-1 lead to a hypofibrinolytic state.
- Sepsis associated coagulopathy is often associated with thrombocytopenia and may warrant further evaluation of conditions present in patients with sepsis including heparin-induced thrombocytopenia, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome.
COVID-19
- Coronavirus disease-2019 (COVID-19) caused by the novel SARS-CoV-2 virus is unique from that traditionally seen in sepsis and has been noted to fluctuate from suppressed fibrinolytic type to enhanced fibrinolytic type DIC. For this reason, frequent monitoring of coagulation and fibrinolytic factors are recommended.
- The rate of complication by DIC is high in COVID-19 nonsurvivors. Thrombotic and hemorrhagic pathologies have both been implicated in these cases of fatality.
- Heparin is the most frequently used anticoagulant used in the treatment of COVID-19 associated thrombosis; however, combination treatment with Nafamostat may be beneficial in the enhanced fibrinolytic type DIC seen in later stages of severe COVID-19 infection.
Leukemia
- In acute leukemias, overexpression of procoagulant molecules including TF on tumor cells have been linked to the development of DIC.
- Administration of cytotoxic chemotherapy agents may increase the release of procoagulant molecules through the process of cell death.
- Chemotherapy agents may further augment coagulation through direct drug interactions with coagulation factors leading to the development of DIC.
Management
• The definitive treatment for DIC is treatment of the underlying clinical condition causing altered coagulation. Management of DIC is primarily supportive and focused on addressing the coagulopathy and organ dysfunction caused by microvascular thrombosis1 (Figure 2).
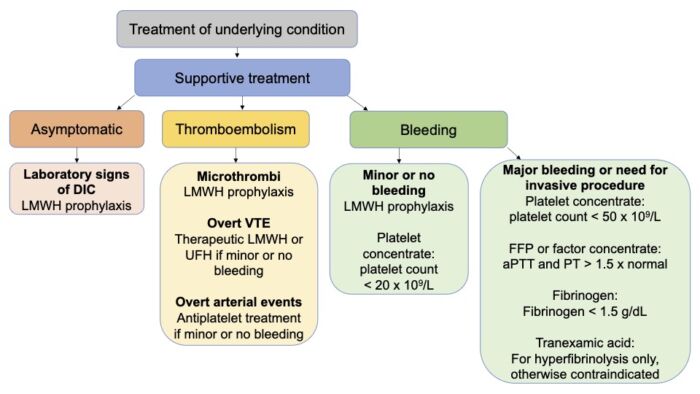
Figure 2. Treatment strategies in DIC. Abbreviations: LMWH = low-molecular weight heparin, UFH = unfractionated heparin, VTE = venous thromboembolism. Adapted from Adelborg K, et al. Disseminated intravascular coagulation: Epidemiology, biomarkers, and management. Br J Haematol. 2021.1
Anticoagulation
- Prophylactic anticoagulation treatment with either unfractionated heparin or low molecular weight heparin in a patient with laboratory signs of DIC or evidence of microvascular emboli.1
- Prophylactic anticoagulation should be held in patient with active bleeding or those with a platelet count less than 20 x 109/L.
- Therapeutic anticoagulation should be used in patients with overt venous thromboembolism.
Platelet Transfusion
- Threshold for platelet transfusion differs based on the clinical condition of the patient and presence or absence of bleeding.
- The recommended threshold for platelet transfusion in patients with major bleeding or at high risk for bleeding is usually 50 x 109/L.
- Threshold for platelet transfusion in patients with minor bleeding or no bleeding is usually 20 x 109/L.
Coagulation Factors
- Coagulation factor administration should be considered for patients with major bleeding or those with laboratory indicators of high bleeding risk such as prolonged PT and aPTT.
- Replacement of coagulation factors can be achieved by fresh frozen plasma transfusion or through coagulation factor concentrate.
- Coagulation factor concentrate may be preferred in cases where there is concern for volume overload.
- Fibrinogen should be maintained at a level of 1.5 g/L which can be achieved with fibrinogen concentrate or cryoprecipitate.
Novel Therapies
- Antithrombin, a serine protease, acts primarily as an anticoagulant through its inhibition of thrombin and activated Factor X. Antithrombin also has antimicrobial and immune system modulating properties that propose a biologic pathway for treatment of sepsis-induced coagulopathy.
- Nafamostat, a serine protease inhibitor, has antifibrinolytic properties useful in the treatment of DIC as well as direct effects on viral transmission by blocking a transmembrane serine protease used by SARS-CoV-2 to infect alveolar epithelial cells. Its use in combination with heparin has been proposed as a potential treatment option for COVID-19 associated coagulopathy.
References
- Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: Epidemiology, biomarkers, and management. Br J Haematol. 2021;192(5):803-18. PubMed
- Papageorgiou C, Jourdi G, Adjambri E, et al. Disseminated intravascular coagulation: An update on pathogenesis, diagnosis, and therapeutic strategies. Clin Appl Thromb Hemost. 2018;24(9_suppl):8S-28S. PubMed
- Taylor FB Jr, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001; 86:1327–30. PubMed
- Asakura H, Takahashi H, Uchiyama T, et al. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2016; 14:42. PubMed
- Giustozzi M, Ehrlinder H, Bongiovanni D, et al. Coagulopathy and sepsis: Pathophysiology, clinical manifestations and treatment. Blood Rev. 2021.50:100864. PubMed
- Asakura, H., Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021; 113: 45–57. PubMed
- Iba T, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology 2020; 132:1238–45. PubMed
- Hayakawa, M. Pathophysiology of trauma-induced coagulopathy: disseminated intravascular coagulation with the fibrinolytic phenotype. J Intensive Care. 2017; 5:14. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.