Copy link
Diabetes Mellitus: Classification, Pathophysiology, Complications, and Management
Last updated: 10/20/2025
Key Points
- Preoperative evaluation of patients with diabetes mellitus (DM) should focus on disease duration and progression, medication compliance, presence of comorbidities, and glycemic control.
- Control of glucose levels during the perioperative period, using subcutaneous or intravenous insulin, is crucial in facilitating wound healing, reducing the risk of ICU admission, and lowering postoperative mortality.
- Neuropathy is a common complication of DM with various types, including distal symmetrical polyneuropathy and diabetic autonomic neuropathy.
Introduction
- DM is defined as a group of metabolic disorders characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both.
- Diagnostic criteria for DM include1-3
- fasting plasma glucose ≥126 mg/dL (7.0 mmol/L);
- a 2-hour plasma glucose ≥200 mg/dL (11.1 mmol/L) during a 75-g oral glucose tolerance test; and
- a random plasma glucose ≥200 mg/dL with symptoms, or HbA1c ≥6.5%.
Epidemiology
- Diabetes is a major global health burden with rapidly increasing prevalence. In 2022, an estimated 828 million adults worldwide had diabetes, with the vast majority (approximately 90–95%) having T2DM.4
- The global age-standardized prevalence in 2021 was 6.1%, and projections indicate that more than 1.3 billion people may have diabetes by 2050.
- In the United States, the prevalence is approximately 11.3% of the population, with significant proportions undiagnosed.
- Diabetes is associated with substantial morbidity, mortality, and economic burden, primarily due to its microvascular and macrovascular complications.4
Classification
- Each subclassification has different causes and management strategies. These subclassifications allow for tailored clinical management for patients.
- Type 1 DM (T1DM) is an autoimmune condition that causes the destruction of insulin-producing β-cells, resulting in insulin deficiency.5
- Type 2 DM (T2DM) is a nonautoimmune condition characterized by the progressive loss of sufficient β-cell insulin secretion over time. Typically, patients exhibit insulin resistance as well as metabolic syndrome.5
- Prediabetes usually precedes T2DM. Patients with prediabetes typically have elevated glucose levels but not high enough to meet criteria for T2DM. These patients have a fasting blood glucose ranging from 100-125 mg/dL or a 2-hour postoral glucose tolerance test glucose of 140-200 mg/dL.6
- GDM occurs when a parturient is diagnosed with DM during the second or third trimester without a prior history or diagnosis of DM.5
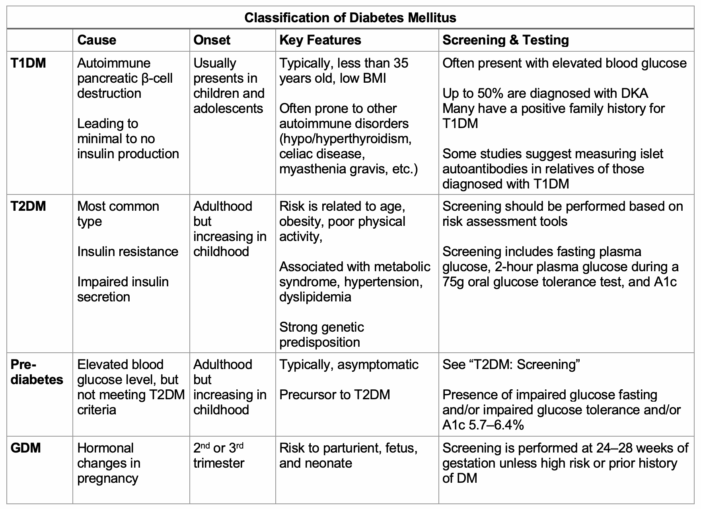

Table 1. Subclassifications of DM based on cause, onset, key features, and screening.5,6 Abbreviations: DM, diabetes mellitus; GDM, gestational diabetes mellitus; OGTT, oral glucose tolerance test.
Pathophysiology
- The pathophysiology of diabetes centers on mechanisms that lead to chronic hyperglycemia, with distinct processes in T1DM and T2DM.1-4
T1DM
- Chronic hyperglycemia results from autoimmune destruction of pancreatic β-cells, leading to an absolute deficiency of insulin.
- Genetically susceptible individuals develop autoreactive T lymphocytes that target β-cell antigens, often triggered by environmental factors such as viral infections.
- The loss of β-cell mass is progressive, and the disease typically manifests when insulin secretion falls below a critical threshold, resulting in unopposed hepatic glucose production and decreased peripheral glucose uptake.
- The American Diabetes Association notes that this autoimmune process is characterized by the presence of islet autoantibodies and is strongly linked to specific HLA haplotypes.
T2DM
- Chronic hyperglycemia arises from a combination of insulin resistance (primarily in muscle, liver, and adipose tissue) and inadequate compensatory insulin secretion by β-cells. Initially, insulin resistance is compensated by increased insulin production.
- Over time, β-cell dysfunction develops due to genetic predisposition, glucotoxicity, lipotoxicity, oxidative stress, and endoplasmic reticulum stress, ultimately leading to insufficient insulin to maintain normoglycemia.
- The American Diabetes Association notes that both insulin resistance and β-cell dysfunction are required for the development of hyperglycemia in T2DM.
- In both types, chronic hyperglycemia itself exacerbates β-cell dysfunction and insulin resistance through mechanisms such as oxidative stress, advanced glycation end-product (AGE) formation, and activation of pro-inflammatory pathways, further perpetuating the disease process.3,4
Long-Term Complications
- DM, when untreated, can lead to long-term complications affecting multiple organ systems. Patients are at risk of cardiovascular, renal, eye, and neurologic disease. These complications include damage to both the micro- and macrovasculature, as well as neuropathy.6,7
- The progression of complications depends on the severity and duration of poor glycemic control, which can be monitored using glycated hemoglobin A1c (HbA1c). Glycation of proteins and lipids occurs in the setting of chronic hyperglycemia, leading to damage to blood vessels. The HbA1c test measures the percentage of glycated hemoglobin, representing the blood glucose levels over the past 2-3 months.6,7
- Examples of long-term complications, categorized by organ system, are listed below.6,7
- Cardiovascular disease is the leading cause of death in patients with DM. Hypertension, peripheral vascular disease, and heart failure are a few examples.
- Chronic kidney disease is commonly seen in this patient population. DM is the leading cause of end-stage renal disease in the United States.
- Diabetic retinopathy is a complication of diabetes in which the blood vessels in the retina are damaged, leading to vision loss and a risk of potential blindness.
- Stroke is a serious neurologic complication of DM. The risk of stroke is twice as high in patients with DM compared to those without DM.
- Diabetic neuropathy (DN) is commonly seen in patients with DM. There are various types of DN, typically classified as symmetric or asymmetric.8
- Examples of symmetric DN include painful autonomic neuropathy, insulin neuritis, and polyneuropathy after ketoacidosis. Examples of asymmetrical DN include asymmetric proximal DN, radiculoplexus neuropathies, median neuropathy at the wrist, and ulnar neuropathy at the elbow.
- Distal symmetrical polyneuropathy and diabetic autonomic neuropathy are important subtypes to discuss.
- Distal symmetrical polyneuropathy is the most common DN, occurring when there is damage to nerves, typically presenting as symmetric, distal extremity neuropathy (glove and stocking distribution).
- DN management includes controlling hyperglycemia and addressing cardiovascular risk factors. Analgesics, non-steroidal anti-inflammatory medications, antidepressants, and anticonvulsants are used for DN.
- Diabetic autonomic neuropathy can be defined as dysfunction of the sympathetic and parasympathetic nervous systems due to poor glycemic control. All autonomically innervated organs are affected, but those most commonly implicated include the eye, bladder, intestine, reproductive organs, sweat glands, and cardiovascular system.
- Diabetic autonomic neuropathy affects multiple organ systems. Hallmark features include orthostatic hypotension, resting tachycardia, gastroparesis, erectile dysfunction, and lack of heart rate response to respiration rate.6,7
- Diabetic autonomic neuropathy management is symptomatic control.
Management
T2DM
- Oral medications are first-line agents to address the pathophysiology of insulin resistance and relative insulin deficiency.
- Metformin is the initial drug of choice due to its efficacy, safety, weight neutrality, and cardiovascular benefits.
- Other oral agents, such as sulfonylureas, SGLT2 inhibitors, DPP-4 inhibitors, and thiazolidinediones, are selected based on comorbidities, risk of hypoglycemia, weight effects, and cost. SGLT2 inhibitors and GLP-1 receptor agonists are preferred in patients with cardiovascular or renal disease due to their proven benefits in these populations.
- As β-cell function declines, combination therapy is often required, and agents are added sequentially or in combination to achieve glycemic targets.
- The American Diabetes Association and the American Association of Clinical Endocrinology recommend individualizing therapy to minimize hypoglycemia and weight gain, while maximizing cardiovascular and renal protection.1-4
T1DM
- Insulin therapy is indicated in:
- T1DM (absolute insulin deficiency)
- T2DM when oral agents fail to achieve glycemic targets
- When there is severe hyperglycemia (A1C >10%, blood glucose ≥300 mg/dL)
- Catabolic symptoms (weight loss, ketosis)
- During acute illness
- Insulin is essential in T1DM due to the autoimmune destruction of β-cells and is titrated to mimic physiologic insulin secretion.
Table 2 below summarizes the most commonly used oral and injectable medications for diabetes management.
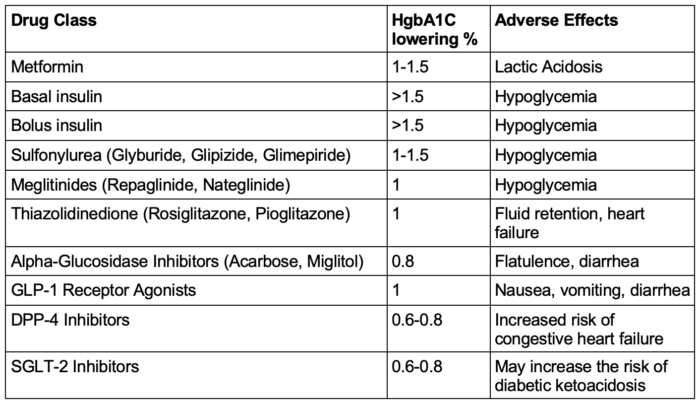
Table 2. Medications commonly used to treat type 2 diabetes. Abbreviations: HgbA1C, hemoglobin A1c; GLP-1, glucagon-like peptide-1; DPP-4, dipeptidyl peptidase-4; SGLT-2, sodium-glucose cotransporter 2. Adapted from Nathan DM. Diabetes: Advances in diagnosis and treatment. JAMA. 2015;314(10):1052-62.9/sup
References
- Diagnosis and classification of diabetes: Standards of care in diabetes-2025. Diabetes Care. 2025;48(Supplement_1): S27-S49. PubMed
- Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2023;46(10):e151-e199. PubMed
- Buysschaert M, Medina JL, Buysschaert B, Bergman M. Definitions (and current controversies) of diabetes and prediabetes. Current Diabetes Reviews. 2016;12(1):8-13. PubMed
- GBD 2021 diabetes collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203-34. PubMed
- American Diabetes Association Professional Practice Committee; 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47 (Supplement_1): S20–S42. PubMed
- Sapra A, Bhandari P. Diabetes. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2025. Accessed date: April 16, 2025. Link
- Dogra P, Anastasopoulou C, Jialal I. Diabetic Perioperative Management. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2025. Accessed date: April 16, 2025. Link
- Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82(964):95-100. Link
- Nathan DM. Diabetes: Advances in diagnosis and treatment. JAMA. 2015;314(10):1052-62. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.