Copy link
Dexmedetomidine
Last updated: 04/18/2024
Key Points
- Dexmedetomidine has sedative, hypnotic, analgesic, and sympatholytic properties without any respiratory depression.
- It is commonly used as a component of general anesthesia, in the intensive care unit (ICU) for sedation in mechanically ventilated patients, for procedural sedation, as an emergence delirium prophylactic agent in pediatric patients, as a preoperative anxiolytic, and as an adjunct to local anesthetics in regional anesthesia and nerve blocks. Most recently, it has been used in the treatment of alcohol withdrawal.
- Dexmedetomidine is a highly selective α2-adrenergic agonist that works at the locus ceruleus to produce a sedated yet arousable patient.
- Adverse effects of dexmedetomidine include dose-dependent hypotension and bradycardia, as well as antimuscarinic effects.
Pharmacology
Trade name- Precedex
Physiochemical Characteristics1
- Dexmedetomidine ((S)-4-[1-(2,2-Dimethylphenylethyl]-3-H-imidazole) is available as a water-soluble salt that is typically diluted to 4 mcg/mL.
Mechanism of Action2
- Dexmedetomidine is a highly selective α2-adrenergic agonist (α2:α1 = 1620:1), similar in nature to clonidine (α2:α1 = 220:1).
- The agonism occurs in the central nervous system; specifically, the α2 receptors on neurons from the locus ceruleus are inhibited, which prevents norepinephrine release in the ventrolateral preoptic nucleus. This activates sleep centers in the brain.
- The disinhibited nucleus reduces arousal in the midbrain, hypothalamic, and pontine nuclei, resulting in a sedated yet easily arousable patient.
Pharmacokinetics2,3
- The pharmacokinetics of dexmedetomidine are listed in Table 1.
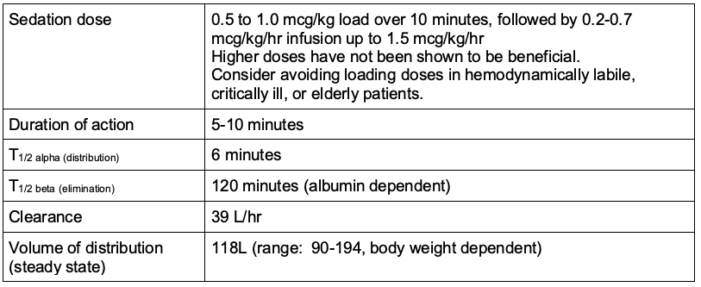
Table 1. Pharmacokinetics of dexmedetomidine
- Absorption
- Only approved for intravenous (IV) use but well absorbed intranasally and via buccal mucosa.
- Oral bioavailability is ~16% due to extensive first-pass effect.
- Redistribution
- Short initial distribution half-life (~6 minutes)
- Readily crosses blood-brain and placental barriers
- Exhibits linear pharmacokinetics in the range of 0.2-0.7 mcg/kg/hr (for up to 24 hours)
- Metabolism
- Undergoes almost complete biotransformation, involving both direct glucuronidation and the cytochrome P450 system with resultant inactive metabolites
- Hepatic extraction ratio = 0.7
- Clearance values are affected by varying levels of hepatic impairment.
- Excretion
- Dexmedetomidine and its metabolites are primarily (95%) eliminated through the renal route via urine.
- Not significantly different in patients with severe renal impairment
- Protein binding
- Exhibits high-protein binding, with approximately 94% bound to plasma proteins
Systemic Effects2,3
The systemic effects of dexmedetomidine administration are listed in Table 2.
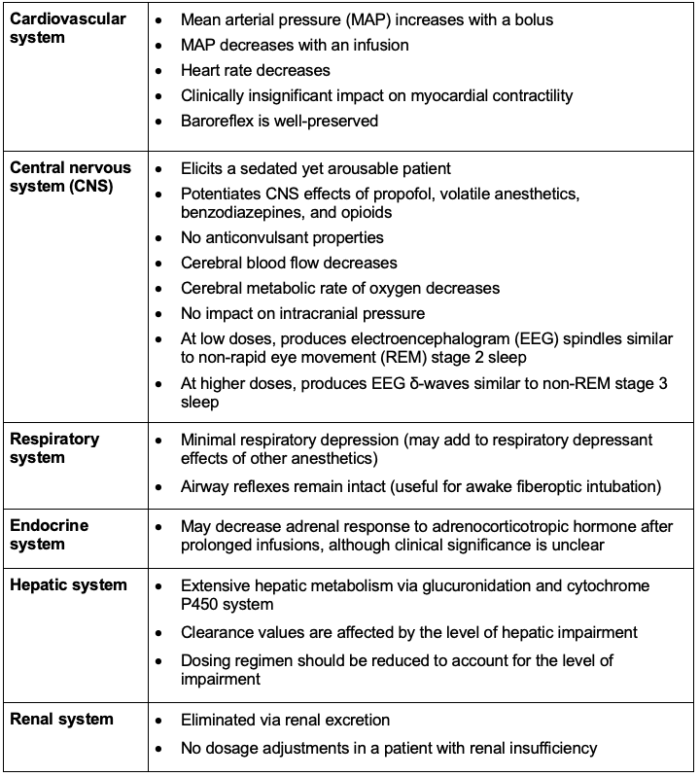
Table 2. Systemic effects of dexmedetomidine administration
Clinical Uses
Component of General Anesthesia1
- Dexmedetomidine may be used as a component of a multimodal anesthetic. It has been demonstrated to decrease the need for other anesthetics, including sevoflurane and propofol.
- It demonstrates both an intra- and postoperative opioid-sparing effect.
Sedation in ICU1
- Although initially approved for only 24 hours of ICU sedation, multiple studies have demonstrated sufficient safety profile through 30 days.
- Its use reduces the duration of mechanical ventilation and the ICU length of stay without a difference in mortality compared to traditional sedatives.4
Sedation for Medical Procedures
- Dexmedetomidine is FDA-approved for therapeutic procedures, diagnostic procedures, and awake fiberoptic intubations.
- Studies demonstrated a significantly reduced incidence of rescue midazolam required to complete the aforementioned procedures relative to placebo.5
Emergence Delirium Prophylaxis in Pediatric Anesthesia
- An IV bolus dose of 0.5 mcg/kg immediately following induction demonstrated a significant reduction in the incidence of emergence delirium, minimal side effects, no delay in emergence, and a reduction in volatile and analgesic requirements.6
Preoperative Anxiolysis
- Intranasal dexmedetomidine 1-2 mcg/kg is an effective anxiolytic premedication, particularly in the pediatric population; however, the clinical effect may not be observed for up to 30-45 minutes.7
Adjunct to Local Anesthetics in Regional Anesthesia
- Perineural dexmedetomidine as an adjutant to local anesthetic nerve blocks increases the duration of the block.8
- Administration of dexmedetomidine via the IV route has a similar efficacy to that of the perineural route.9
Adverse Effects
- Antimuscarinic effects may occur due to α2 adrenal receptor-mediated inhibition of acetylcholine release.
- Dexmedetomidine causes dose-dependent hypotension and bradycardia due to stimulation of presynaptic alpha receptors, leading to a decreased norepinephrine release and a decrease of central sympathetic flow.
- Hypertension can occur with fast administration or with a loading dose due to stimulation of alpha subtype receptors on the vascular smooth muscles. It is usually self-limiting and does not require treatment.
- No effective antagonist is available for human use.
Cautions
- In patients with baseline bradycardia, dexmedetomidine can worsen bradycardia.
- In patients with hypotension, dexmedetomidine can worsen hypotension.
- Consider avoiding a bolus in frail, critically ill, and elderly patients.
- In patients with heart failure, there is evidence of possible exacerbation of myocardial dysfunction.10
References
- Weerink MAS, Struys MMRF, Hannivoort LN, et al Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. PubMed
- Abola R, Geralemou S, Szafran M, et al. Intravenous anesthetics. In: Barash PG, et al. (editors). Clinical Anesthesia. Philadelphia, Wolters Kluwer/Lippincott Williams & Wilkins, 2017: 496-8.
- Young ER. Clinical Anesthesia Procedures of the Massachusetts General Hospital, 6th Edition. Anesth Prog. 2005 Spring;52(1):45.
- Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev. 2015;1(1):CD010269. PubMed
- Bergese SD, Candiotti KA, Bokesch PM, et al; AWAKE Study Group. A phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther. 2010;17(6):586-95. PubMed
- Manning AN, Bezzo LK, Hobson JK, et al. Dexmedetomidine dosing to prevent pediatric emergence delirium. AANA J. 2020;88(5):359-64. PubMed
- Zhang X, Bai X, Zhang Q, et al. The safety and efficacy of intranasal dexmedetomidine during electrochemotherapy for facial vascular malformation: a double-blind, randomized clinical trial. J Oral Maxillofac Surg. 2013;71(11):1835-42. PubMed
- Wu HH, Wang HT, Jin JJ, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9(3): e93114. PubMed
- Abdallah FW, Dwyer T, Chan VW, et al. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: A randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology. 2016 Mar;124(3):683-95. PubMed
- Page RL, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: A scientific statement from the American Heart Association. Circulation. 2016;134(12): e261. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.