Copy link
D-Transposition of the Great Arteries
Last updated: 03/14/2023
Key Points
- In d-Transposition of the great arteries (d-TGA), the aorta and pulmonary artery (PA) are switched in anatomic position so that the aorta arises from the right ventricle (RV) and the PA arises from the left ventricle (LV).
- The systemic and pulmonary circulations are arranged in parallel rather than in series, resulting in cyanosis and a systemic RV.1
- Mandatory mixing via intracardiac shunting is necessary for survival.2
- Historically, a surgical atrial switch was done to redirect deoxygenated blood toward the lungs and oxygenated blood toward the body. Currently, a definitive repair with the arterial switch operation (ASO) is done in the neonatal period, allowing for a systemic LV.
Introduction
- There are two types of transposition of the great arteries (TGA):
- Dextro-TGA (d-TGA) is the more common form and is a cyanotic lesion.
- Levo-TGA (l-TGA, also called congenitally corrected-TGA [cc-TGA]) is rare in occurrence, non-cyanotic, and will be discussed in a separate summary.
Epidemiology
- The prevalence of d-TGA is approximately 3.71 per 10,000 births, with a male: female ratio of 2-3:1. d-TGA is the second most common congenital cardiac lesion, accounting for 6% of all congenital heart disease, and 20% of cyanotic heart disease.
Embryology
- Septation and rotation of the conotruncal cushions are necessary to align the aorta with the LV and the PA with the RV. Mesenchymal cells derived from the neural crest aid in this process; however, in d-TGA, mutations in the nodal signaling pathway disrupt normal neural crest migration and the conotruncal septum fails to follow a spiral course.3
Genetic Factors
- Chromosomal associations are uncommon but include heterotaxy and DiGeorge syndrome. About 10% have relatives with congenital heart disease. The sibling recurrence rate has been reported to be as high as 2%.
Associated Anomalies
- d-TGA frequently occurs as an isolated lesion but may be associated with other cardiac anomalies, including ventricular septal defects (VSD), left ventricular outflow obstruction, pulmonary stenosis, mitral and tricuspid valve abnormalities, and abnormal coronary artery anatomy.
Anatomy
- d-TGA is characterized by atrio-ventricular concordance and ventriculo-arterial discordance. In d-TGA, the aorta is anterior and rightward of the PA.
- Deoxygenated systemic blood returns to the right atrium (RA) where it then passes through the RV into the aorta. The aorta carries the blood to the body.
- On the other side, oxygenated blood returns to the left atrium (LA) via the pulmonary veins (PVs). It then passes through the LV to the discordant PA and back to the lungs.
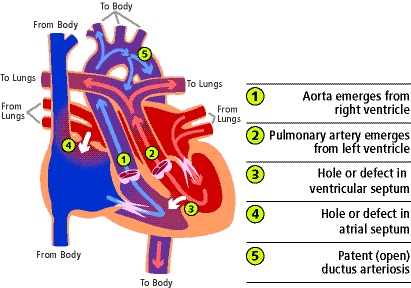
Figure 1. Anatomical diagram of d-TGA with mixing via an ASD, VSD, and PDA. Used with permission from the University of Michigan Health, C.S. Mott Children’s Hospital.
Pathophysiology
- The systemic and pulmonary circulations are arranged in parallel, and intercirculatory mixing must occur to sustain postnatal life. Mixing can occur via:
- patent ductus arteriosus (PDA);
- at the atrial level with a patent foramen ovale (PFO) or atrial septal defect (ASD);
- at the ventricular level with a VSD (~45% of d-TGA patients); or
- via bronchopulmonary collaterals.
- The degree of mixing (and thus the degree of effective pulmonary and systemic blood flow) depends on the number, size, and location of the anatomic communications as well as the compliance of the atria, ventricles, and vascular beds in the systemic and pulmonary circuits. An unrestrictive atrial-level shunt is typically best.
- In the presence of restrictive shunts (such as when the PDA and PFO close in the absence of a VSD), the patient may develop unacceptable hypoxemia, anaerobic metabolism, lactic acidosis, and shock. If uncorrected, the lesion is fatal.
- Reverse differential cyanosis (i.e., preductal saturation less than postductal arterial saturation) may occur when the pulmonary vascular resistance (PVR) is higher than the pulmonary artery pressure.
Diagnosis
Prenatal Diagnosis
- Since 2013, fetal CHD screening has included the evaluation of the outflow tracts.
- Antenatal screening only identifies about 40-75% of cases.
Postnatal Diagnosis
- Postnatal hypoxemia is the typical presentation.
- After ductal closure, patients may present with cyanosis, lactic acidosis, and shock.4
- Newborn pulse oximetry can help identify those not diagnosed in utero; unexplained postnatal hypoxemia (SpO2 less than 95%), +/- murmur, warrants echocardiography.
Diagnostic Studies
- Chest radiograph: The classic finding is cardiomegaly with narrow superior mediastinum because the PA is posterior to the aorta. The heart appears like an “egg on a string.”
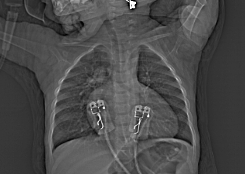
Figure 2. Classic “egg on a string” appearance of the heart in a patient with dTGA. Case courtesy of Mohammad A. ElBeialy. Radiopaedia.org, rID:35361. Link.
- Echocardiogram: Parallel rather than intertwined great vessels with PA arising from LV.
- Cardiac catheterization: Along with hemodynamic data, including pulmonary vascular resistance, specific emphasis on coronary artery anatomy is helpful for surgical planning.
- Computed tomography (CT) angiography: Abnormal great vessel and coronary anatomy may be directly visualized; function can be assessed with cardiac-gated cine CT.
Treatment Strategies
Medical Management5
- If diagnosed in utero, delivery should occur a center with balloon atrial septostomy (BAS) capabilities.
- Ductal patency may be maintained by continuous prostaglandin E1 (PGE1) infusion.
- Supplemental O2, intubation, and neuromuscular blockade may be necessary.
- For the neonate in shock, inotropic support, and/or extracorporeal membrane oxygenation may be needed.
Interventional Management
- In the setting of a restrictive ASD/PFO, BAS allows for increased intercirculatory mixing.
- BAS may be somewhat elective to allow for the discontinuation of PGE1 and/or a delayed surgical repair or it may be urgent/emergent in the setting of hypoxemia.
- Risks include arrhythmias, vascular or atrial trauma, and cardiac tamponade.
Surgical Management
• Operative repair now typically happens in the first weeks of life. Waiting at least a few days allows the transition from fetal to neonatal circulation, PVR to decrease, end-organ function to improve, and time to evaluate for other anomalies. Delaying surgery increases the risk of the risk of neurologic injury.
Atrial Switch Operation
- Historically, d-TGA was surgically repaired with an atrial level “switch” – either the Mustard (synthetic baffle material) or the Senning (native atrial tissue baffle material) (Figure 2).
- The goal of the atrial switch is to provide the patient with an intra-atrial baffle that will direct deoxygenated blood from the RA to the PA via the LV and oxygenated blood returning from the lungs from the LA to the aorta via the RV.
- The atrial switch establishes a series circulation with a systemic RV.
- Long-term sequelae of an atrial switch include RV failure, arrhythmias, tricuspid valve dysfunction, baffle-related problems, and sudden death.
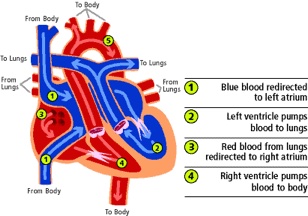
Figure 3. Diagram depicting d-TGA anatomy following the atrial switch operation. Used with permission from the University of Michigan Health, C.S. Mott Children’s Hospital.
Arterial Switch Operation (ASO)
- The ASO is currently the standard of care for d-TGA, and the goal is to reposition the great vessels into a normal anatomic arrangement (Figure 3).
- The aorta and PA are transected above the sinotubular junction and reattached to the correct outflow tracts. Specifically, the aorta is reattached to the former pulmonary (now neoaortic) root. The PA is brought anterior to the aorta (called a Lecompte maneuver) and reattached at the former aortic root. The coronary buttons are excised and reimplanted into the neoaortic root.
- The ASO establishes a series circulation with the LV as the systemic ventricle.
- Complications of the procedure are coronary artery obstruction, aortic insufficiency, and pulmonary stenosis.
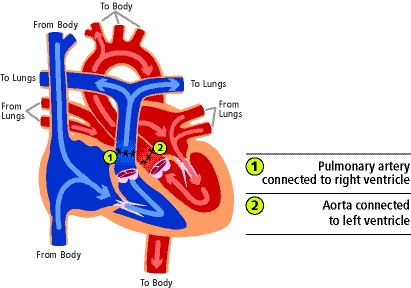
Figure 4. Diagram depicting d-TGA anatomy following arterial switch operation. Used with permission from the University of Michigan Health, C.S. Mott Children’s Hospital.
Anesthetic Considerations for Arterial Switch Operation
Preoperative
History and Physical
- General: gestational age, genetic abnormalities, and perinatal organ system dysfunction
- Special considerations for patients with TGA: Baseline hemodynamics and inotrope requirement, arterial oxygen saturation, presence of reverse differential cyanosis
Labs
- Complete blood cell count, type and crossmatch, metabolic panel, +/- mixed venous oxygen saturation, and lactate.
Imaging/Diagnostics
- ECG, echocardiogram, +/- CT, hemodynamic catherization.
- Coronary angiography if coronary anatomy cannot be adequately seen on echo.
- +/- Head ultrasound to rule out intraventricular hemorrhage before heparinization.
Intraoperative
Monitoring
- Standard American Society of Anesthesiologists monitors (pre-/post-ductal oxygen saturation, 5-lead ECG)
- Near-infrared spectroscopy
- Invasive pressure monitoring – central venous, arterial, and left atrial (placed intra-op) lines
- Transesophageal or epicardial echocardiogram
Vascular Access
- 1-2 peripheral intravenous catheters (PGE1 requires dedicated access), bubble filters
- Central line (peripheral placement by anesthesia v. central placement by surgeon)
Hemodynamic Goals
Induction and Pre-Cardiopulmonary Bypass (CPB)
- Goals are to maintain adequate cardiac output (via maintenance of heart rate, contractility, and preload) and arterial saturation (i.e., intercirculatory mixing).6
- Goal saturations are typically greater than 75%. FiO2 can be up-titrated in the setting of de-saturation unresponsive to volume administration or adjustments to ventilation, keeping in mind that high FiO2 may increase pulmonary flow at the expense of systemic flow.
- Increases in pulmonary vascular resistance and decreases in systemic vascular resistance should be avoided as they lead to decreased pulmonary blood flow and reduced mixing.
- The immature myocardium has a limited reserve and will be sensitive to anesthesia-induced myocardial depression; inotropic support may be needed prior to bypass.
- Prostaglandins should be continued until CPB.
Post-CPB
- The goal of the post-CPB period is to maintain adequate cardiac output while avoiding left atrial and pulmonary hypertension.
- Excessive volume administration is avoided as it could lead to LV overdistention, LA hypertension, and potential coronary compression.
- Post-CPB myocardial dysfunction is due to long CPB times with inadequate myocardial protection, coronary insufficiency (air vs. anatomic), and/or the acute increase in afterload for the newly systemic LV. Inotropic support and/or afterload reduction is generally needed.
- Postrepair echocardiography must assess the adequacy of coronary perfusion given that coronary button excision/reimplantation onto the neoaorta may cause coronary insufficiency.
- Extensive suture lines, long CPB time, and an immature coagulation system often result in severe post-CPB bleeding.
Postoperative
- Immediate concerns include bleeding, low cardiac output syndrome, coronary insufficiency, and volume overload.
References
- Villafañe F, Lantin-Hermoso MR, Bhatt AB, et al. D-transposition of the great arteries: The current era of the arterial switch operation. J Am Coll Cardiol. 2014;64(5):498– 511. PubMed
- Hiremath G, Natarajan G, Math D, et al. Impact of balloon atrial septostomy in neonates with transposition of great arteries. J Perinatol. 2011; 31:494. PubMed
- London B. Ciliary genes causing transposition of the great arteries? Not silly at all. Circ Res. 2020; 126:822–3. PubMed
- Blyth, M. Howe, D, Gnanapragasam J, et al. The hidden mortality of transposition of the great arteries and survival advantage provided by prenatal diagnosis. BJOG 2008; 115:1096. PubMed
- Komarlu R, Morell VO, Kreutzer J, et al. Dextro-transposition of the great arteries (D-TGA). In: Munoz R., Morell V, da Cruz E, Vetterly C, da Silva J. (eds) Critical Care of Children with Heart Disease. Springer, Cham. (2020) 351-368. Link
- Latham GJ, Joffe DC, Eisses MJ, et al. Anesthetic considerations and management of transposition of the great arteries. Semin Cardiothorac Vasc Anesth. 2015;19(3):233-42. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.