Copy link
Cryoprecipitate
Last updated: 08/17/2023
Key Points
- Cryoprecipitate is a derivative of fresh frozen plasma (FFP) containing primarily factor VIII, factor XIII, fibrinogen, von Willebrand factor (vWF), and fibronectin.
- The primary perioperative use of cryoprecipitate is in patients with acquired fibrinogen deficiency with fibrinogen levels less than 80-100 mg/dL (0.8 – 1 g/L) or in the presence of excessive bleeding when fibrinogen cannot be measured.
- A pool of 10 cryoprecipitate units raises the plasma fibrinogen levels by approximately 100 mg/dL (1 g/L).
- Cryoprecipitate has been withdrawn from use in most European countries secondary to safety concerns and has been replaced by fibrinogen concentrates. Currently, the U.S. Food and Drug Administration (FDA) only approves fibrinogen concentrates for congenital fibrinogen deficiency.
Introduction
- Cryoprecipitate was first introduced in the 1960s by Pool and colleagues to treat patients with von Willebrand disease (vWD) and hemophilia A.1
- Currently, cryoprecipitate is primarily used to replenish fibrinogen levels in patients with acquired fibrinogen deficiency, such as trauma, cardiac surgery, liver transplantation, obstetric hemorrhage, etc.
Production
- Cryoprecipitate is derived from FFP. A unit of FFP is first thawed and then centrifuged for a variable amount of time, depending on institutional practices. The supernatant is removed, and the resulting precipitate is isolated and refrozen, representing one unit of cryoprecipitate.1
- Cryoprecipitate is stored at -18℃ and has a shelf-life of 12 months.1
- Each unit of cryoprecipitate contains:2
- Factor VIII
- Factor XIII
- Fibrinogen
- Fibronectin
- vWF
- Platelet microparticles
- Per the US FDA and the Association for Advancement of Blood and Biotherapies standards, the minimum levels of each component is as follows:3
- Fibrinogen – more than 150 mg; most units contain 250-300 mg of fibrinogen
- Factor VIII – more than 80 international units
- Factor XIII – 50-75 units
- vWF – 100-150 units
- Before administration, the product is thawed and used immediately. If not used immediately, it can be stored at 20-24℃ and must be used within 4-6 hours.1
- Typically, 5-10 units of cryoprecipitate are pooled, depending on specific institutional practices.
- A pool of 10 cryoprecipitate units raises the plasma fibrinogen levels by approximately 100 mg/dL.4
- When administering cryoprecipitate, there is variability in practice regarding the need for ABO and Rh compatibility.
- Cryoprecipitate has 3-10 times lower plasma volumes than fresh frozen plasma or platelets, and therefore, the risk of hemolysis from ABO-incompatible cryoprecipitate would be expectedly low.5
- ABO compatibility is recommended when administering cryoprecipitate in neonates, small children, solid organ, and hematopoietic cell transplant recipients. Rh compatibility is generally not needed secondary to the low volume of plasma.3
Clinical Uses
- Cryoprecipitate is primarily indicated for the management of bleeding in patients with acquired fibrinogen deficiency, as may occur in the following settings:
- Trauma, massive hemorrhage
- Obstetric hemorrhage
- Cardiac surgery
- Liver disease
- Professional societies differ on guideline recommendations for use and specific dosing regimens of cryoprecipitate. The American Society of Anesthesiologists provides the following recommendations:6
- Cryoprecipitate is indicated when fibrinogen concentration is less than 80-100 mg/dL in the presence of excessive bleeding or when fibrinogen concentrations cannot be measured in a timely fashion.
- Cryoprecipitate is rarely indicated if fibrinogen concentration is greater than 150 mg/dL in nonpregnant patients.
- Pregnant patients require higher fibrinogen levels for adequate coagulation. The normal fibrinogen level in a term pregnancy is 350 – 650 mg/dL, which is nearly double that of nonpregnant adults (200-400 mg/dL). A target fibrinogen level greater than 200 mg/dL is commonly recommended for pregnant patients with postpartum hemorrhage.7
- Cryoprecipitate is not recommended as first-line treatment of congenital factor deficiencies such as congenital hypofibrinogenemia, vWD, factor XIII deficiency, or hemophilia A (factor VIII deficiency) due to safety reasons and the easy availability of specific coagulation concentrates, but it may be used in the absence of specific recombinant or plasma-derived factor concentrates.3
Cryoprecipitate vs. Fibrinogen Concentrate8
- Fibrinogen concentrate is produced from human plasma and lyophilized or freeze-dried, thereby providing a stable, easily reconstituted formulation for rapid administration. It has a longer shelf life (3 years) compared with cryoprecipitate (1 year).
- Fibrinogen concentrate provides several advantages over cryoprecipitate, including a more predictable dose when reconstituted, reduced risk of viral transmission due to chemical inactivation in its preparation, and a reduced risk of immunological transfusion reaction.
- The efficacy (or noninferiority) of fibrinogen concentrate compared with cryoprecipitate is a topic of ongoing, active research. The efficacy of fibrinogen concentrate in certain clinical scenarios involving acquired coagulopathy (e.g., cardiac procedures utilizing cardiopulmonary bypass) may be limited given the absence of VWF, Factor XIII, and fibronectin normally found in cryoprecipitate.
- Fibrinogen concentrate, per dose, is generally more expensive.
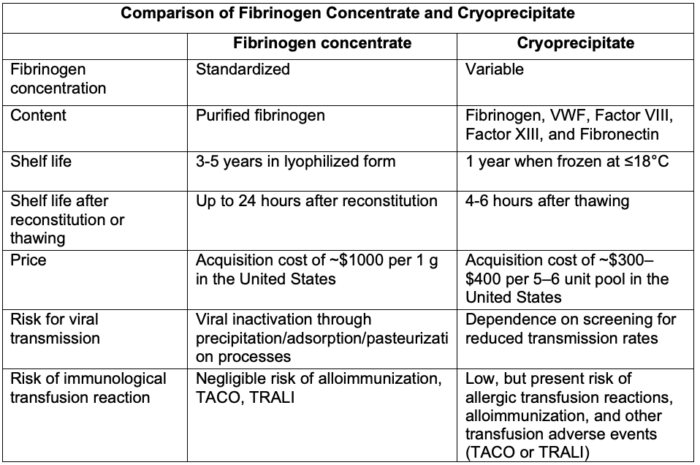
Table 1. Comparison of fibrinogen concentrate and cryoprecipitate. Abbreviations: TACO, transfusion-associated circulatory overload; TRALI, transfusion-related acute lung injury; VWF, von Willebrand factor. Adapted from Hensley N, Mazzeffi MA. Pro-con debate: Fibrinogen concentrate or cryoprecipitate for treatment of acquired hypofibrinogenemia in cardiac surgical patients. Anesth Analg. 2021.8
Complications
- Complications from cryoprecipitate administration are rare, similar to other blood component therapy.
- The risks of cryoprecipitate administration include:3
- Transmission of infections – approximately equivalent infection risk as a unit of red blood cells. However, since many units of cryoprecipitate are pooled, the risk per dose depends on the number of units in the pool.
- Transfusion-associated circulatory overload (TACO) – lower risk than FFP
- Transfusion-related acute lung injury (TRALI) – lower risk than FFP
- Allergic transfusion reaction, alloimmunization, etc. – lower risk than FFP
References
- Callum JL, Karkouti K, Lin Y. Cryoprecipitate: the current state of knowledge. Transfus Med Rev. 2009; 23: 177-88. PubMed
- Longnecker DE, Mackey SC, Newman MF, Sandberg WS, Zapol WM. eds. Anesthesiology, 3e. McGraw Hill; 2017.
- Tobian A. Clinical use of cryoprecipitate. In Post T (ed). UpToDate. 2023. Link
- Levy JH, Shaz B. Using plasma and plasma component therapy. In: Kaushansky K, Prchal JT, Burns LJ, Lichtman MA, Levi M, Linch DC (Eds). Williams Hematology, 10e. McGraw Hill; 2021. Accessed March 05, 2023. Link
- Dunbar NM. Time to stop worrying about ABO incompatible cryoprecipitate transfusions in adults. Transfusion. 2021;61(1):1-4. PubMed
- Practice guidelines for perioperative blood management: An updated report by the American Society of Anesthesiologists Task Force on perioperative blood management. Anesthesiology. 2015; 122:241–75. PubMed
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care consensus No. 7: Placenta accreta spectrum. Obstet Gynecol. 2018;132(6): e259-75. PubMed
- Hensley N, Mazzeffi MA. Pro-con debate: Fibrinogen concentrate or cryoprecipitate for treatment of acquired hypofibrinogenemia in cardiac surgical patients. Anesth Analg. 2021;133(1):19-28. PubMed
Other References
- MCL Education. The Secrets of Cryoprecipitate: A Blood Banking Process. Mayo Clinic Laboratories. Accessed March 5, 2023. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.