Copy link
Congenitally Corrected Transposition of the Great Arteries
Last updated: 08/01/2023
Key Points
- In congenitally corrected TGA (cc-TGA), the morphologic left ventricle (LV) is anatomically on the right, and the morphologic right ventricle (RV) is on the anatomic left.
- The systemic and pulmonary circulations are arranged in series, but there is both atrioventricular and ventriculoarterial discordance and a systemic right ventricle (RV).
- In the absence of a concurrent cyanotic lesion, patients with cc-TGA will be fully saturated, although arrhythmias and eventual RV failure are common.
- Current management options are controversial and include: 1. no operative intervention; 2. physiologic palliation with repair of accompanying defects but leaving a systemic RV; and 3. anatomic double switch operation (DSO) with restoration of systemic LV.
Introduction
- There are two types of transposition of the great arteries (TGA):
- Dextro-TGA (d-TGA) is the more common form and is a cyanotic lesion (see OA Summary D-Transposition of the Great Arteries).
- Levo-TGA (l-TGA, also called cc-TGA) is rare in occurrence and noncyanotic.
Epidemiology
- Incidence: Very rare, less than 1% of all congenital heart lesions, and less than 10,000 total patients in the USA
Embryology
- During cardiogenesis, normal looping of the heart to the right occurs in the third week of development. With cc-TGA, the heart loops to the left, which leads to ventricular inversion.
Genetic Factors
- Familial recurrence and consanguinity are associated with an autosomal recessive mechanism, but the exact etiology is currently unknown.
Associated Anomalies
- More than 90% of patients have a concomitant lesion.
- Lesions include ventricular septal defect (VSD), left ventricular outflow tract obstruction (which is morphologic, right-sided and may be at multiple levels), Ebsteinoid tricuspid valve abnormality, and atrioventricular (AV) conduction system abnormalities.
Anatomy
- cc-TGA is characterized by atrioventricular and ventriculoarterial discordance such that the ventricles, along with their associated atrioventricular valve (AVV), are inverted. The morphologic LV will have the mitral valve inflow, and the morphologic RV will have the tricuspid valve inflow.
- L-TGA is “congenitally corrected” because the circulation is in series and blood flows in the “correct” physiologic direction:
- Deoxygenated systemic blood returns to the heart via the vena cava into the right atrium (RA) and then passes through the mitral valve and the LV into the pulmonary artery (PA) to the lungs.
- Oxygenated blood returns from the lungs via the pulmonary veins in the left atria (LA) and then passes through the tricuspid valve into the RV and out through the aorta to the body.
- The circulation has a systemic RV and tricuspid valve.
- The aorta is anterior and leftward of the PA.
- The coronary anatomy is a mirror image of normal.
- The AV node is in an abnormal location and conduction abnormalities are common.
- Notes on nomenclature (morphologic vs. anatomic):
- Morphologic LV refers to the cavity that meets the criteria for the definition of LV (e.g., two papillary muscles, bicuspid AVV, apex forming). With cc-TGA, the morphologic LV is on the right side of the heart.
- Anatomic LV refers to the cavity on the left side of the body or heart. With cc-TGA, the anatomic left ventricle is the RV.
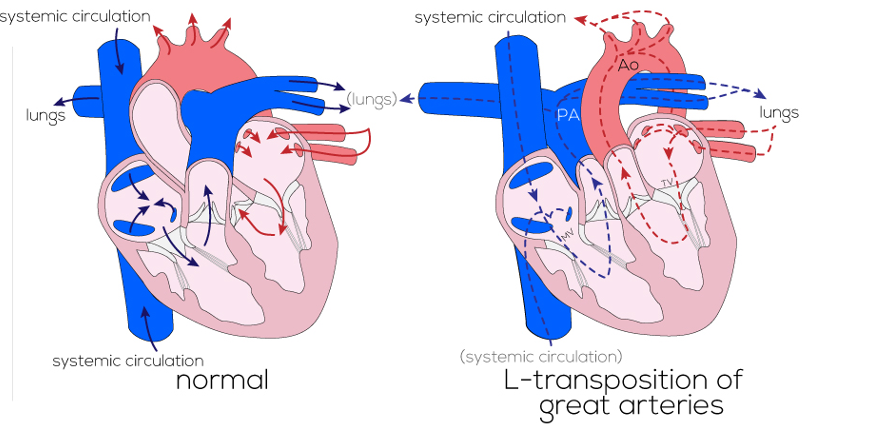
Figure 1. Diagram of anatomy and blood flow in the normal versus the L-TGA (cc-TGA) heart. Used with permission from the Atlas of Human Cardiac Anatomy, University of Minnesota/© Medtronic. Link.
Pathophysiology
- Since blood travels in the normal anatomic direction with this lesion, patients without concomitant lesions or arrhythmias may not come to clinical attention until the systemic RV and/or tricuspid valve (TV) begins to fail.
- The RV is not teleologically designed to withstand high afterload. As RV function diminishes and the ventricle dilates, the TV may become increasingly insufficient.
- Abnormal/dysplastic (Ebsteinoid) TVs are associated with systemic AVV regurgitation.
- Conduction abnormalities, including complete AV block, are common and often progressive.3
- Patients are acyanotic unless there is an accompanying cyanotic lesion, e.g., pulmonary stenosis and a VSD with right to left shunt (from morphologic LV to morphologic RV).
- Life expectancy is shortened.
Diagnosis
Presentation
- For most patients, signs and symptoms are due to concomitant cardiac defects.
- Early presentation may occur with cyanosis from pulmonary stenosis and a VSD.
- Without associated defects, patients may present later in adulthood with a failing systemic RV and TV or with arrhythmias or heart block due to intrinsic abnormalities in the conduction system.
Diagnostic Studies
- Echocardiography: Important for assessment of ventricular function, outflow tract obstruction, AVV regurgitation, and location of other lesions (e.g., VSDs)
- Cardiac catheterization and cardiac magnetic resonance imaging: May provide additional anatomic information as well as hemodynamic data
Treatment Strategies
Medical Management
- Pharmacologic treatment may be needed for concomitant lesions and/or heart failure.
Interventional Management
- Permanent pacemaker placement is indicated for high-degree AV blocks.
Surgical Management
- There is currently no consensus regarding the optimal management of cc-TGA, and treatment remains institution-dependent. Surgical options are no operation, physiologic repair, or anatomic repair.
- No operation
- Operative intervention does not guarantee better quality or a longer duration of life.
- The risk of not intervening includes RV and/or TV failure, need for transplantation, and early death.
- Physiologic repair
- A physiologic repair is one that addresses concomitant lesions but leaves a systemic RV. Examples: VSD closure, relief of outflow tract obstruction
- Complications: RV and/or TV failure
- Anatomic repair
- Anatomic repair results in a systemic LV with atrioventricular and ventriculoarterial concordance.
- This is done with a double switch operation (DSO) (atrial switch and arterial switch, Figure 2).
- The ultimate goal is to redirect atrial flow to the appropriate ventricle (atrial switch to establish atrioventricular concordance) and to redirect ventricular outflow to the correct great artery (arterial switch to establish ventriculoarterial concordance).
- Preconditioning with a PA band may be a prerequisite to a DSO. Without preconditioning, the sudden increase in afterload may cause the LV to acutely fail. The initial band is tightened to achieve LV pressure (LVP) greater than 50% systemic pressure with normal LV function. The final goal is LVP greater than 80% of SBP prior to DSO. Pulmonary stenosis may serve as natural preconditioning for the morphologic LV.
- Complications: bleeding, AV conduction block, cavoatrial obstruction, coronary insufficiency, aortic insufficiency, pulmonary stenosis, and LV failure
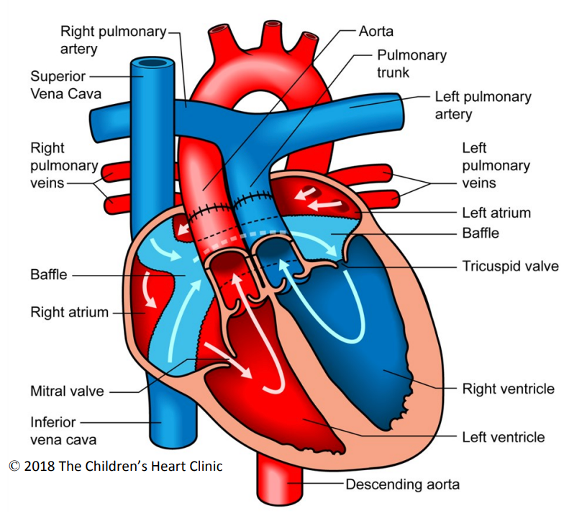
Figure 2. Double switch operation. The surgically placed atrial baffle directs deoxygenated blood to the left side of the heart (TV and RV) and oxygenated blood to the right side of the heart (MV and LV). The aorta and PA are transected above the sinotubular junction and reattached to the correct outflow tracts. The coronary buttons are excised and reimplanted into the neoaortic root. Used with permission from the Children’s Heart Clinic, Children’s Minnesota.
Anesthetic Considerations for Interventional/Surgical Management3
Preoperative
- Preoperative assessment includes a thorough understanding of the patient’s complicated anatomy. Specifically, it is important to know:
- presence of associated defects, prior operations, residual defects;
- signs and symptoms of CHF or cyanosis;
- pacemaker settings (if applicable);
- systemic RV function and TV pathology; and
- if prior PA band, then functional status of LV and degree of success of retraining.
- Labs – complete blood cell count, type & crossmatch
- ECG – evaluate conduction system abnormalities
- Imaging – echocardiography +/- computed tomography scan, magnetic resonance imaging, and catheterization data
Intraoperative
Monitoring
- Standard American Society of Anesthesiologists monitors with 5-lead ECG
- Near-infrared spectroscopy
- Invasive pressure monitoring – central venous, arterial, and left atrial (placed intraoperatively) lines
- Transesophageal or epicardial echocardiogram
Vascular access
- 1-2 peripheral intravenous catheters, bubble filters if atrial septal defect or VSD
- Central line
Intraoperative management
Induction and Pre-Cardiopulmonary Bypass
- Adequate cardiac output should be maintained. Ionotropic support may be needed in the setting of RV (systemic ventricle) failure and/or prior PA banding.
- Pacemaker settings should be re-set (DOO) to allow for the use of electrocautery.
- Pacing should be readily available for patients without a permanent pacemaker.
- The choice of induction and maintenance anesthetic agents will depend on the patient’s cardiac function and the palliation or repair being performed.
- Patients presenting for DSO after PAB placement are at risk for vascular or cardiac injury during reoperative sternotomy and may require massive hemorrhage and/or emergent peripheral cannulation.
Post-CPB
- A left atrial pressure line and epicardial pacing leads are placed near the end of CPB.
- Long CPB times may result in stunned myocardium with depressed ventricular function requiring inotropic support. This dysfunction may be especially significant in a suboptimally retrained LV and compounds pre-existing RV dysfunction. Some patients may be unable to separate from CPB and need to be transitioned to ECMO in the OR.
- Coronary artery insufficiency: Rerouting and reimplantation of the coronary arteries may result in kinking and ischemic myocardium with regional wall motion abnormalities.
- Extensive surgical repair and long CPB times may result in severe bleeding. Filtered autologous blood is retransfused to the patient after protamine administration. Point-of-care testing with ACT, viscoelastography, and quantitative platelet counts aid in hemostasis management. Blood products are typically needed to correct coagulation defects.
• Pharmacologic agents that may aid hemostasis include fibrinogen concentrate, recombinant Factor VIIa or Prothrombin Complex Concentrate (PCC).4
• Conduction abnormalities: Arrhythmias may need pharmacologic intervention and iatrogenic heart block is common and may require epicardial pacing.
• Central venous pressure should be closely monitored when atrial level baffles are created, given the risk for baffle obstruction and resultant SVC syndrome.
Postoperative
• Immediate DSO complications include bleeding, LV failure, conduction abnormalities requiring a permanent pacemaker, aortic insufficiency, and/or intra-atrial baffle obstruction.
References
- Devaney EJ, Bove EL. Congenitally corrected transposition of the great arteries. In: Mavroudis C, Backer CL (Eds). Pediatric Cardiac Surgery. West Sussex, UK. John Wiley & Sons, Ltd: 530-41.
- Kumar TKS. Congenitally corrected transposition of the great arteries. J Thorac Dis. 2020;12(3):1213-1218. PubMed
- Baruteau AE, Abrams DJ, Ho SY, et al. Cardiac conduction system in congenitally corrected transposition of the great arteries and its clinical relevance. J Am Heart Assoc. 2017;6(12): e007759 PubMed
- Guzzetta NA, Williams GD. Current use of factor concentrates in pediatric cardiac anesthesia. Paediatr Anaesth. 2017;27(7):678-687. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.