Copy link
Cerebral Autoregulation
Last updated: 01/03/2023
Key Points
- Cerebral autoregulation is the ability to maintain a constant cerebral blood flow (CBF) even with changes in cerebral perfusion pressure (CPP).
- Autoregulation of CBF consists of interactions among myogenic, neurogenic, metabolic, and endothelial mechanisms.
- Physiologic, pathologic, and anesthetic conditions can cause increases or decreases in CBF.
Introduction
- Cerebral autoregulation is the ability of the body to maintain a constant CBF despite changes in CPP.1
- Cerebral autoregulation protects the brain from hypo- or hyperperfusion secondary to decreases or increases in CPP, respectively.
- Traditionally, it was believed that a constant CBF is expected in healthy adults within a mean arterial pressure (MAP) range of 60-160 mmHg, or CPP of 50-150 mmHg, assuming a normal intracranial pressure (ICP) of 10 mmHg. CPP equals MAP minus ICP or central venous pressure, whichever is higher (Figure 1).1 The limits of cerebral autoregulation in infants and children are not known and autoregulation probably occurs at lower absolute values than in adults.
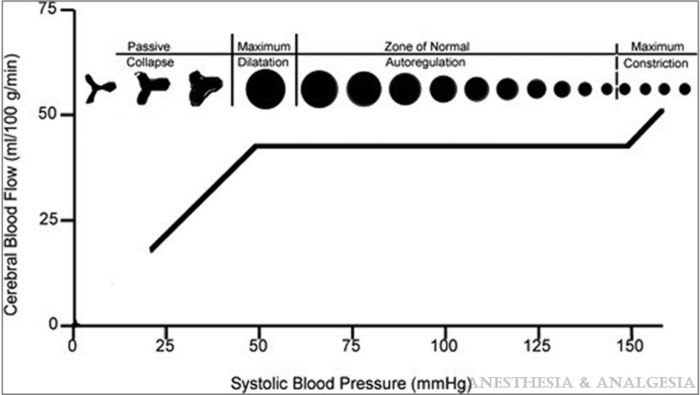
Figure 1. Graphical representation of the relationship between cerebral blood flow and mean arterial blood pressure in the normal brain. Reproduced from White H, Venkatesh, B. Cerebral perfusion pressure in neurotrauma: a review. Anesthesia & Analgesia. 2008; 107(3): 979-88.
- However, recent studies question this wide range of MAP and the shape of the proposed curve. Instead, it appears that the brain adapts more easily to very slow changes and transient increases in CPP than to sudden decreases.
- The properties of cerebral autoregulation are dynamic and distinct for each individual and cannot be confidently predicted. Therefore, CBF surrogates (such as cerebral blood velocity, cerebral oximetry, and electroencephalogram [EEG]) are often closely monitored in situations where significant hemodynamic fluctuations are expected.2
- Techniques for bedside assessment of cerebral autoregulation, including transcranial Doppler (TCD), near-infrared spectroscopy (NIRS), brain tissue oxygen monitor, and ICP-derived pressure reactivity (PRx), among others, allow for individualized MAP management in critically ill patients and might lead to better outcomes.3
Mechanism
- Autoregulation of CBF involves the interaction of the myogenic, neurogenic, metabolic, and endothelial mechanisms through different physiologic and pathologic states.
- The myogenic response involves contraction of small arteries and arterioles in response to increased CPP and conversely, relaxation of the arteriolar tone with decreases in CPP.
- The neurogenic mechanism involves the release of vasoactive neurotransmitters in response to depolarization of interneurons.3
- Metabolic regulation consists of reflex vasodilation or constriction of the microvasculature secondary to changes in the local partial pressure of CO2. Increased perfusion of a vascular bed temporarily increases blood flow, flushing out CO2. This leads to localized reflex vasoconstriction, with a reduction of blood flow until CO2 returns to its normal partial pressure. Conversely, decreased perfusion leads to increased CO2, resulting in localized vasodilation and restoring blood flow to the affected vascular territory.
- The endothelial mechanism works to release nitric oxide (vasodilator), thromboxane A2, and endothelin-1 (vasoconstrictors) to help regulate CBF.
- Neurovascular coupling is the reciprocal relationship between cerebral metabolic rate for oxygen (CMRO2) and CBF.
Other Physiologic Parameters That Affect CBF
- CO2: When PaCO2 is within the range of 25 and 70 mmHg, it correlates with CBF. One mmHg increase in PaCO2 will result in 1-2 mL/100g/min (or 4%) increase in CBF. Similarly, reductions in PaCO2 cause decreases in CBF. The effect of PaCO2 changes in CBF is attenuated after 6 hours. Prolonged hyperventilation should be avoided secondary to the risk of cerebral ischemia.
- O2: Decreases in O2 do not influence CBF until a critical threshold of 50-60 mmHg is reached, below which there is a marked increase in CBF.4
- Temperature: Hypothermia results in a dose-dependent reduction in CMRO2. For each 1°C reduction in temperature, CMRO2 decreases by 6-7%. The reduction in CMRO2 continues beyond the point where the EEG is completely suppressed (at 18–20°C). Hyperthermia, conversely, increases CMRO2 up to 42°C, when the toxic effects of hyperthermia result in progressive decreases in CMRO2.4
- Glucose: Severe hypoglycemia (blood glucose <2 mmol/L or 36 mg/dL) results in increased CBF.
Effects of Anesthetic Agent on CBF
- Intravenous anesthetics: Intravenous agents reduce both CMRO2 and CBF and are considered cerebral vasoconstrictors and reduce CMRO2 and CBF in parallel, except for ketamine, which causes increases in CMRO2 and CBF.
- Opioids: Opioids slightly decrease CMRO2 and CBF.
- Volatile agents: Volatile agents produce a dose-dependent reduction of CMRO2. However, due to their intrinsic vasodilatory properties (isoflurane=desflurane>sevoflurane), volatile agents uncouple CMRO2 and CBF. Their overall effect on CBF will result in a balance between CMRO2-related CBF reduction and intrinsic vasodilatory-related increase in CBF.
- At 0.5 MAC, CBF is reduced.
- At 1.0 MAC, CBF level is at baseline.
- Above 1.0 MAC, CBF is increased.
- N2O: N2O administration results in increased CMRO2 and CBF. Its effect on CBF is attenuated when administered with volatile agents and is minimal when administered with propofol.4
Common Pathologic States That Affect Cerebral Autoregulation
- Traumatic brain injury: Cerebral autoregulation is impaired or absent in severe traumatic brain injury. In these patients, decreases in CPP result in decreases in CBF that may reach ischemic levels and lead to secondary brain injury.1
- Cerebral tumors: Cerebral tumors are frequently associated with disruption of the blood-brain barrier, leading to the development of vasogenic edema and increase in ICP. Cerebral autoregulation in peritumoral areas is impaired, and these areas are more susceptible to ischemia.
- Chronic hypertension: With hypertension, the cerebral autoregulation curve shifts to the right. Blood pressure management should aim to keep the MAP within 20-25% of baseline if not monitoring autoregulation specifically.4
- Preeclampsia: Endothelial dysfunction, alterations in cerebral blood flow, and impaired autoregulation are some of the mechanisms of cerebral edema in preeclampsia.5
References
- Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016;34(3):465-77. PubMed
- Brassard P, Labrecque L, Smirl JD, et al. Losing the dogmatic view of cerebral autoregulation. Physiol Rep. 2021;9(15): e14982. PubMed
- Rivera-Lara L, Geocadin R, Zorrilla-Vaca A, et al. Near-infrared spectroscopy derived cerebral autoregulation indices independently predict clinical outcome in acutely Ill comatose patients. J Neurosurg Anesthesiol. 2020;32(3):234-41. PubMed
- Patel PM, Drummond JC, Lemkuil BP. Cerebral physiology and the effects of anesthetic drugs. In: Gropper M, Eriksson L, Fleisher L, Wiener-Kronish J, Cohen N, Leslie K. Miller’s anesthesia. 8th Edition. Philadelphia; PA. Elsevier; 2015: 294-332.
- Bergman L, Cluver C, Carlberg N, et al. Cerebral perfusion pressure and autoregulation in eclampsia—a case control study. Am J Obstet Gynecol. 2021;225(2):185. e1-185. e9. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.