Copy link
Postoperative Nausea and Vomiting in Adults
Last updated: 10/05/2023
Key Points
- Without prophylactic intervention, approximately one-third of patients who have inhalational anesthesia are likely to develop postoperative nausea and vomiting (PONV).
- PONV can lead to prolonged stays in the postanesthesia care unit (PACU), unanticipated hospital admissions, increased risk of aspiration, and discomfort for the patient.
- Risk factors can be categorized as patient-related, anesthetic-related, and surgical factors.
- At least two antiemetic medications should be administered to each patient; medications with different mechanisms of action should be used to enhance effectiveness.
Introduction
- PONV affects around one-third of patients who receive inhalational anesthetics without prophylaxis, though estimates range from as low as 10% to as high as 80%.1
- PONV can lead to prolonged stays in the postanesthesia care unit (PACU), unanticipated hospital admissions, increased risk of aspiration, and discomfort for the patient.2
- It is estimated that an episode of vomiting prolongs PACU stay by about 25 minutes.3
Risk Factors
- Risk factors for PONV in adults can be categorized as surgical, anesthetic, or patient-related factors1,2 (Figure 1).
- Patient-related
- Nonsmoker
- Female gender
- History of motion sickness and/or PONV
- Postoperative opioid use
- Young age (< 50 years old)
- Anesthesia-related
- Volatile anesthetics
- Nitrous oxide
- Higher doses of perioperative opioids
- Surgery-related
- Long duration of surgery
- Type of surgery (abdominal, gynecological, cholecystectomy)
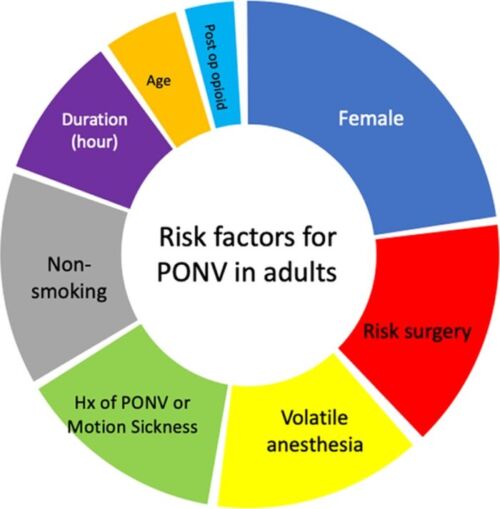
Figure 1. Risk factors for PONV in adults. The size of each segment is proportional to the odds ratios of PONV associated with each factor.
Abbreviations: PONV, postoperative nausea and vomiting; Hx, history
Used with permission from the American Society for Enhanced Recovery.
Risk Stratification
- The simplified Apfel score can be used to predict the patient’s risk for PONV. 0,1, 2, 3, and 4 risk factors correspond to PONV risks of approximately 10%, 20%, 40%, 60%, and 80% respectively.2
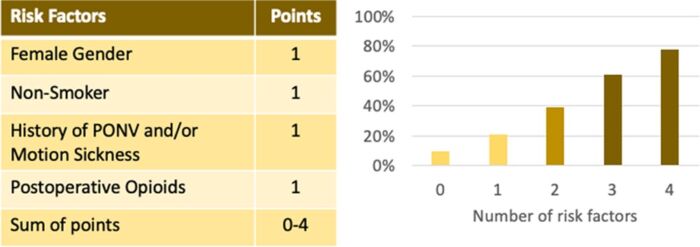
Figure 2. Simplified Apfel score to predict an adult patient’s risk for PONV.
Abbreviation: PONV, postoperative nausea and vomiting
Used with permission from the American Society for Enhanced Recovery.
Prophylaxis
- To reduce the baseline risk of PONV, several strategies may be considered:2
- Regional anesthesia should be performed or analgesic blocks used as a part of a multimodal pain management plan.
- If general anesthesia is necessary, using a propofol-based total intravenous anesthetic (TIVA) should be considered and volatile anesthetics should be avoided.
- Nitrous oxide exposure (less than 1 hour) and opioids should be minimized.
- Sugammadex reversal should be considered instead of neostigmine.
- Adequate hydration of patients undergoing same-day surgery should be ensured.
- For patients with one or two risk factors, at least two antiemetic medications are recommended; three to four antiemetic medications may be beneficial if more than two risk factors are present.
- Aprepitant (Neurokinin 1 (NK1) receptor antagonist) has been reported to have high effectiveness, with decreased need for rescue antiemetics and longer duration of PONV relief.4 It is more effective at the prevention of postoperative vomiting than nausea.2

Figure 3. Prevention and management of PONV in adults.
Abbreviation: PONV, postoperative nausea and vomiting
Used with permission from the American Society for Enhanced Recovery.
Medications to Treat PONV
- Five neurotransmitter receptor sites are important in the vomiting reflex: M1 (muscarinic), D2 (dopamine), H1 (histamine), 5-hydroxytryptamine-3 (5HT3) (serotonin), and NK1 (substance P).5
- The area postrema is an important site for M1, D2, 5HT3, and NK1 receptors; the vestibular nucleus is associated with H1 receptors; and vagal afferent neurons are associated with 5HT3 receptors.5
- Medications used to prevent and treat PONV interact with many different receptor targets, and often a single agent will interact with more than one receptor:
- NK1 receptor antagonists (e.g., aprepitant)
- Targets the nucleus tractus solitaries and area postrema
- Side effects: aprepitant is a moderate inhibitor of CYP3A4; rolapitant inhibits CYP2D6.5
- 5HT3 receptor antagonists (e.g., ondansetron, granisetron)
- Targets the area postrema
- Side effects: mild headache (most frequent), lightheadedness, dizziness, constipation, QTc prolongation5
- Antihistamines (e.g., hydroxyzine, diphenhydramine, promethazine)
- Side effects: sedation
- Corticosteroids (e.g., dexamethasone)
- Exact mechanism unknown; thought to inhibit prostaglandins peripherally and antagonize serotonin centrally3
- Side effects: insomnia, increased energy, mood changes5
- Propofol
- Antiemetic mechanism unknown; thought to be due to direct depressant effect on the chemoreceptor trigger zone and vagal nuclei6
- Dopamine antagonists (e.g., metoclopramide, prochlorperazine)
- Targets the area postrema
- Side effects: extrapyramidal effects and dystonia (more seen with the pediatric population)3
- Anticholinergics (e.g., scopolamine, promethazine)
- Antagonizes muscarinic receptors of the vestibular apparatus and the nucleus of the tractus solitarus3
- Side effects: sedation, dry mouth, visual disturbance5
- Phenothiazines (e.g., promethazine, prochlorperazine)
- Antagonize D2-dopamine receptors in the area postrema of the midbrain; also have M1-muscarinic and H1-histamine blocking effects5
- Side effects: sedation, extrapyramidal effects, hypotension5
- Butyrophenones (e.g., droperidol, haloperidol)
- Antagonize central dopaminergic receptors3
- Side effects: sedation, agitation, extrapyramidal effects, hypotension
- Black box warning: risk of prolonged QTc and torsades de pointes
- Benzamides (e.g., metoclopramide, domperidone)
- Antagonize central and peripheral dopaminergic receptors; weak 5HT3 blockade at higher doses
- Side effects: anxiety, restlessness, depression, hyperprolactinemia, QT interval prolongation5
- Domperidone is a D2 blocker with selective peripheral activity in the upper GI tract; it does not cross the blood-brain barrier and lacks central nervous system side effects associated with metoclopramide.5
- Black box warning: risk of irreversible tardive dyskinesia with high dosing and long-term use5
- Prokinetics (e.g., metoclopramide)
- Increases gastrointestinal tract motility, decreases gastric emptying time and gastric volume, increases lower esophageal sphincter tone3
- Vasopressors (e.g., ephedrine)
- May prevent nausea associated with hypotension3
- NK1 receptor antagonists (e.g., aprepitant)
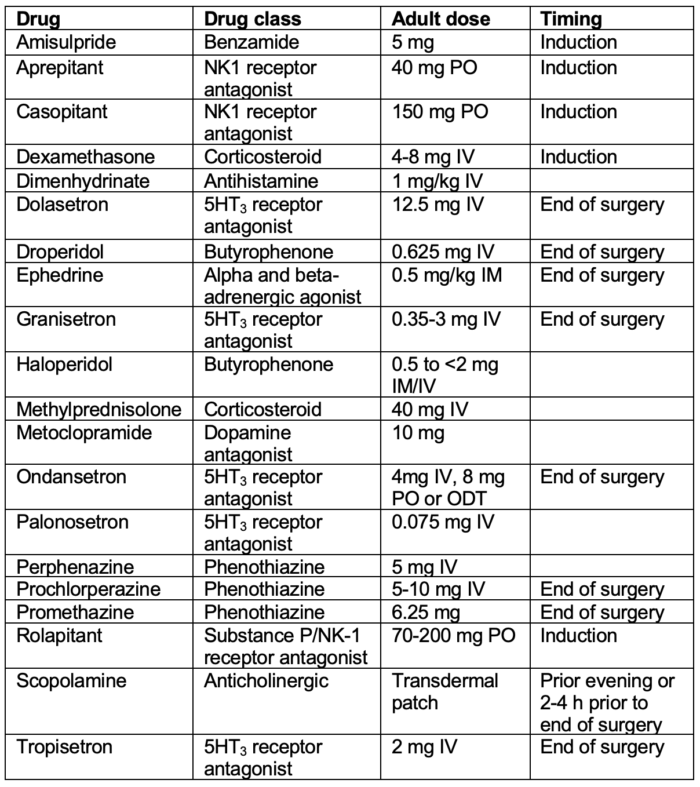
Table 1. Antiemetic doses and timing for prevention of PONV in adults.
Abbreviation: PONV, postoperative nausea and vomiting; PO, per os; IV, intravenous; IM, intramuscular
Adapted from Gan TJ, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020; 131(2): 411-48.2
Rescue Treatment
- If a patient experiences PONV, the same medications used for prophylaxis may be used as rescue treatment.
- Rescue antiemetics should be of a different mechanism of action than the agents given as prophylaxis.
- Reversible causes for nausea and vomiting should be considered:2
- Excessive opioids
- Blood in pharynx
- Bowel obstruction
References
- Braehler MR, Mizrahi I. Postanesthesia recovery. In: Manuel Pardo. Miller's Basics of Anesthesia. Eight edition. Philadelphia, PA; Elsevier; 2023: 697-715.
- Gan, TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020; 131(2): 411-48. PubMed
- Chatterjee, S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol Res Pract. 2011; 2011: 748031. PubMed
- Singh PM, Borle A, Rewari V, et al. Aprepitant for postoperative nausea and vomiting: a systemic review and meta-analysis. Postgraduate Medical Journal. 2016; 92: 87-98. PubMed
- Longstreth GF, Hesketh PJ, Characteristics of antiemetic drugs. UpToDate; 2023. Link
- Folino TB, Muco E, Ssafadi AO, et al. Propofol. In: StatPearls [Internet]. Treasure Island, FL; StatPearls Publishing; 2023. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.