Copy link
Cerebral Vasospasm
Last updated: 01/03/2023
Key Points
- Cerebral vasospasm is a reversible narrowing of the cerebral arteries and arterioles that typically occurs as a complication of aneurysmal subarachnoid hemorrhage (SAH).
- Vasospasm can lead to delayed neurological ischemia and can potentially cause irreversible neurological deficits.
- Current management includes pharmacological prophylaxis (nimodipine), treatment with volume resuscitation to normovolemia and forced hypertension, and endovascular therapy. Management with “triple-H therapy” (hypervolemia, hypertension, and hemodilution) is not recommended.
- Anesthetic management for endovascular therapy must consider the hypotensive effects of anesthetics and cardiopulmonary pathology (e.g., stress-induced cardiomyopathy, pulmonary edema).
Introduction and Clinical Significance
- Cerebral vasospasm is a delayed but temporary narrowing of the cerebral arterial blood vessels and most commonly involves the large proximal vessels from the circle of Willis.
- Vasospasm most commonly occurs between 3-14 days after aneurysmal SAH but may also occur after arteriovenous malformation rupture, nonaneurysmal SAH, traumatic brain injury, or secondary to inflammatory conditions.1,2
- Delayed cerebral ischemia (DCI) is the most concerning complication. DCI presents as clinical deterioration and is associated with cerebral infarction and mortality.1.3
- DCI refers to the clinical or radiographic evidence of ischemia, while vasospasm refers to the radiographic evidence of arterial narrowing. Older literature used the term vasospasm to refer to both the arterial narrowing and the delayed ischemia of SAH.
- Despite modern advances in the management of SAH, cerebral vasospasm and DCI remains a significant potentially preventable cause of morbidity and mortality in the setting of aneurysmal SAH.1,4 (See Aneurysmal Subarachnoid Hemorrhage Part 1: Epidemiology, Pathophysiology, Diagnosis, Cardiac Complications Link)
Pathophysiology
Several mechanisms have been described for the pathophysiology of cerebral vasospasm.
- The breakdown products of hemoglobin can cause the release of oxidative radicals, vasoconstrictors such as endothelin 1, and nitric oxide scavengers. Overall, these molecules promote an environment of vasoconstriction and endothelial damage.1,4
- Hemoglobin is also thought to trigger the release of calcium in smooth muscle, which further promotes the vascular smooth muscle contraction linked to vasospasm.4
- Evidence also suggests a role of inflammatory-mediated remodeling and narrowing of the arterial wall may be involved in the development of vasospasm.1,4
Clinical Presentation
- While angiographic vasospasm is present in approximately 60-90% of patients with SAH, only approximately 30% of these patients will have symptoms.1-3
- Clinical suspicion for DCI arises if there is a change in the level of consciousness (e.g., progression to somnolence and stupor), headache, confusion, or a new focal neurological deficit occurs. Symptomatic vasospasm usually has a gradual onset and runs a progressive course. The neurological deficits are referrable to the territory that has become ischemic.4
- Middle cerebral artery (MCA) vasospasm: monoparesis or hemiparesis and aphasia (if the dominant hemisphere is affected).
- Anterior cerebral artery vasospasm: leg weakness, confusion, drowsiness, poverty of speech, and eventually abulia.
- Vertebrobasilar vasospasm: more generalized neurological deterioration with a reduced level of consciousness.
- Other causes of neurological deterioration, such as elevated intracranial pressure (ICP), hydrocephalus, rebleed, hypoxemia, or electrolyte disturbances (hyponatremia) must be excluded.5
Diagnosis
- The gold standard for diagnosing vasospasm is digital subtraction angiography (Figure 1).
- Computed tomography (CT) angiography combined with CT perfusion mean transit time is commonly used and allows for the direct measurement of arterial narrowing and assessment of perfusion.1
- Transcranial Doppler (TCD) is a useful bedside noninvasive screening and diagnostic tool that measures blood flow velocity in the arteries to detect vasospasm.1,4,5 TCD is best used to detect proximal vasospasm, and it doesn’t reliably detect vasospasm in the more peripheral arteries. TCD mean flow velocities greater than 200 cm/second are usually indicative of severe vasospasm.4
- Electroencephalogram has also been used to predict vasospasm 24 to 72 hours prior to clinical DCI by monitoring for decreasing alpha/delta ratio and decreasing alpha variability. It is most useful in patients with higher grade SAH and poor clinical exams.
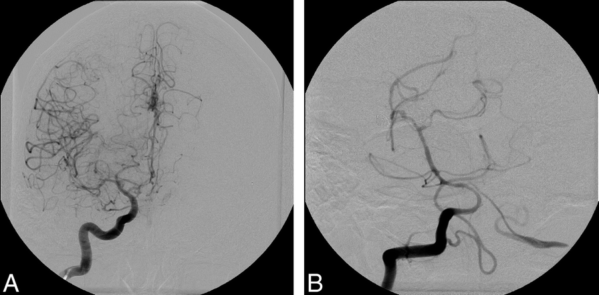
Figure 1. Cerebral vasospasm seen in multiple vascular territories following injection of right internal carotid artery (A) and vertebral artery (B). Used with permission from Farag E, et al. Anesth Analg. 2006;103(6):1618-20.
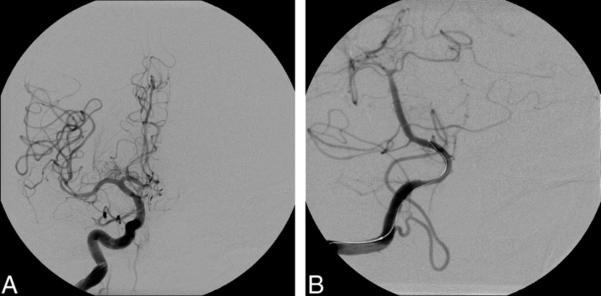
Figure 2. After endovascular treatment utilizing angioplasty, all vessels are free from vasospasm. Used with permission from Farag E, et al. Anesth Analg. 2006;103(6):1618-20.
Treatment- Hypertensive Therapy, Endovascular Therapy
- Oral nimodipine is recommended for vasospasm and DCI prevention in all patients with SAH. Nimodipine has been shown to decrease DCI and improve neurological outcomes.3,4
- Symptomatic cerebral vasospasm has historically been treated with “triple H therapy” which comprises hypervolemia, hypertension, and hemodilution. While effective in reversing vasospasm, triple H therapy is associated with many complications (e.g., pulmonary edema), and its use is no longer supported by current evidence.
- Current management includes fluid resuscitation to euvolemia and induced hypertension (hyperdynamic therapy) after the aneurysm has been secured.2,3 Systolic blood pressure (SBP) goals of 180-220 mmHg or cerebral perfusion pressures greater than 80 mmHg are common.
- Endovascular therapy utilizing balloon angioplasty or intraarterial (IA) vasodilators (verapamil, nicardipine, papaverine, or nitroprusside)2,3 (Figure 2) may be used for vasospasm refractory to hyperdynamic therapy or as the first line in some centers.
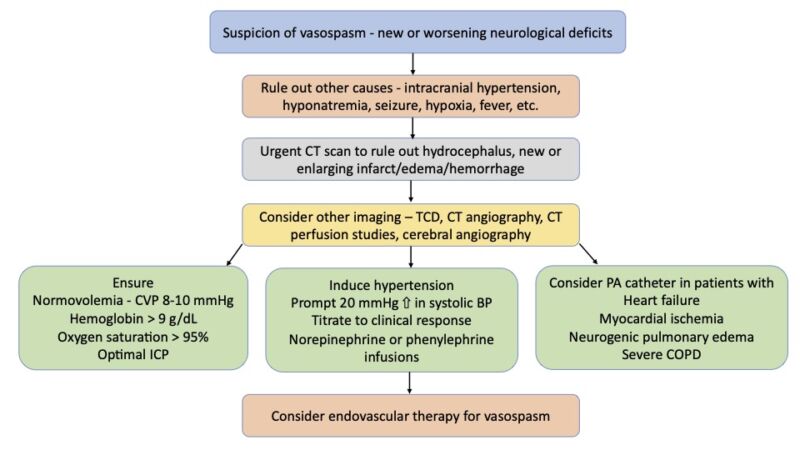
Figure 3. Management of vasospasm. Adapted from Findlay JM, et al. Cerebral Vasospasm: A Review. Can J Neurol Sci. 2016;43(1):15-32.4
Anesthetic Considerations
- Patients are often already intubated and general endotracheal anesthesia is usually recommended (e.g., need for airway protection, uncooperative patient, etc.).
- Ongoing intensive care management should be continued.
- Normovolemia (i.e., central venous pressure 8-10 mmHg or pulmonary capillary wedge pressure 14-16 mmHg) should be maintained.4
- Physician should maintain the hemoglobin greater than 9 g/dl, avoid hypoxia, and optimize the ICP.4
- Hyperdynamic therapy with pressors or inotropes should be maintained. Permissive or forced hypertension to SBP of 220 mmHg is acceptable if the bleeding source has been secured and if there are no cardiopulmonary concerns.
- Endovascular therapy for vasospasm
- Judicious fluid management can help reduce volume overload, pulmonary edema, and worsening of cardiac failure.
- Some systemic circulation of the IA vasodilators is expected. Be prepared to increase the vasopressor infusion or give bolus doses of vasopressors and inotropes to maintain SBP at preoperative target.
- Stress-induced cardiomyopathy (Takotsubo cardiomyopathy, neurogenic stunned myocardium) is common in patients with SAH, and inotropes should be administered to optimize cardiovascular function. Verapamil and other cardiac depressant medications are likely best avoided in these situations.
References
- Singh J, Wicks RT, Wilson JA, et al. Radiographic vasospasm and clinical (symptomatic) vasospasm. In: Ringer AJ, ed. Intracranial Aneurysms. Academic Press; 2018:161-78.
- Sharma D. Perioperative management of aneurysmal subarachnoid hemorrhage Anesthesiology. 2020;133(6):1283-1305. PubMed
- Farag E, Witek AM, Bain M. Intracranial aneurysms: Vasospasm and other issues. In: Fleisher LA, Rosenbaum SH. Complications in Anesthesia. Third ed. Philadelphia Pennsylvania: Elsevier; 2018: 248-55.
- Findlay JM, Nisar J, Darsaut T. Cerebral Vasospasm: A Review. Can J Neurol Sci. 2016;43(1):15-32. PubMed
- Rao GS, Muthuchellappan R. Cerebral vasospasm: current understanding. Curr Opin Anaesthesiol. 2016;29(5):544-51. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.